Abstract
A 55-year-old woman with a history of type 2 diabetes mellitus, hypertension, smoking, and arteriosclerosis underwent urgent coronary intervention for acute myocardial infarction (AMI). One hour post-procedure, she exhibited deteriorating consciousness, tachypnea, and confusion. Clinical evaluation revealed a hyperosmolar hyperglycemic state (HHS) with metabolic acidosis, which likely contributed to her altered mental status. This case highlights the importance of vigilant monitoring for metabolic complications in patients with diabetes following acute cardiac events. Early recognition and multidisciplinary management are critical to improving outcomes in such scenarios.
Keywords
type 2 diabetes mellitus, hyperosmolar hyperglycemic state, acute myocardial infarction, metabolic surveillance, altered mental status, critical care, cardiovascular emergencies
Abbreviations
AMI: acute myocardial infarction; HHS: hyperosmolar hyperglycemic state; ABG: arterial blood gas; TAPSE: tricuspid annular plane systolic excursion; IVC: inferior vena cava; PCI: post-percutaneous coronary intervention; DKA: diabetic ketoacidosis
Introduction
Acute myocardial infarction (AMI) is a life-threatening condition requiring urgent intervention. However, postprocedural complications, particularly in patients with diabetes, can increase morbidity and hospitalization rates [1, 2]. Hyperosmolar hyperglycemic state (HHS) is a severe diabetes complication characterized by extreme hyperglycemia, hyperosmolality, and dehydration, often leading to altered mental status [3, 4]. While metabolic complications following AMI are well-documented, HHS remains an underrecognized yet life-threatening consequence requiring immediate intervention. This case report discusses the clinical presentation, diagnosis, and management of a patient who developed HHS following urgent coronary intervention for AMI.
Case Presentation
A 55-year-old woman with a history of type 2 diabetes mellitus, hypertension, smoking, and arteriosclerosis presented with AMI symptoms. She underwent urgent coronary intervention, which was performed successfully. However, one-hour post-procedure, she exhibited a decline in consciousness, tachypnea, and confusion. Her medications included metformin (1000 mg), insulin (sliding scale), bisoprolol (5 mg), ramipril (5 mg), rosuvastatin (20 mg), aspirin (100 mg), and clopidogrel (75 mg). No known drug allergies were reported.
Clinical examination
- Respiratory: Tachypnea (24–28 breaths/min), snoring, high tidal volume. SpO₂: 93% (room air).
- Cardiovascular: Pulse 95 bpm, BP 110/65 mmHg, cold extremities, capillary refill time: 3 seconds.
- Neurological: Confusion, responsive to pain (AVPU: P). No focal deficits.
- Abdomen: Generalized tenderness, stiff abdomen.
- Temperature: 37.1°C.
Laboratory findings
Blood tests revealed severe hyperglycemia (45 mmol/L) and hyperosmolality (365 mOsm/L), supporting an HHS diagnosis (Table 1). Arterial blood gas (ABG) revealed metabolic acidosis with a pH of 7.3, a base excess of -8 mmol/L, and lactate of 2.1 mmol/L, suggesting lactic acidosis (Table 2).
| Parameter | Value | Reference range | Interpretation |
| White blood cells (WBC) | 16.6 × 10⁹/L | 4.0–11.0 × 10⁹/L | Elevated (inflammatory response) |
| Hemoglobin (Hb) | 145 g/L | 120–160 g/L | Normal |
| Platelets (PLT) | 255 × 10^9/L | 150–450 × 10^9/L | Normal |
| Creatinine | 155 µmol/L | 45–90 µmol/L | Elevated (renal impairment) |
| Blood urea nitrogen (BUN) | 18 mM | 2.5–7.1 mM | Elevated |
| Sodium (Na) | 148 mmol/L | 135–145 mmol/L | Elevated (hypernatremia) |
| Potassium (K) | 3.4 mmol/L | 3.5–5.0 mmol/L | Borderline |
| Troponin T | 185 ng/L | < 14 ng/L | Elevated (AMI) |
| Blood glucose (BG) | 45 mmol/L | 4.0–6.0 mmol/L | Severely elevated (HHS) |
| Serum osmolality | 365 mOsm/L | 275–295 mOsm/L | Elevated (HHS) |
| Urine ketones | Absent | Negative | Absence confirms HHS over DKA |
Table 1: Key laboratory results demonstrating metabolic derangements in a case of post-AMI hyperosmolar hyperglycemic state. HHS: hyperosmolar hyperglycemic state; AMI: acute myocardial infarction; DKA: diabetic ketoacidosis.
| Parameter | Value | Reference range | Interpretation |
| pH | 7.3 | 7.35–7.45 | Acidosis |
| pO2 | 85 mmHg | 80–100 mmHg | Mild hypoxemia |
| pCO2 | 25 mmHg | 35–45 mmHg | Respiratory alkalosis (compensatory) |
| Bicarbonate (HCO3-) | 21 mmol/L | 22–26 mmol/L | Metabolic acidosis |
| Base excess (BE) | -8 mmol/L | -2 to +2 mmol/L | Significant metabolic acidosis |
| Lactate | 2.1 mmol/L | 0.5–1.6 mmol/L | Elevated (lactic acidosis) |
| Oxygen saturation (SaO2) | 93% | 95–100% | Mild hypoxemia |
Table 2: Arterial blood gas (ABG) findings in a patient with HHS and metabolic acidosis. pH: potential of hydrogen; pO₂: partial pressure of oxygen; pCO₂: partial pressure of carbon dioxide.
Imaging studies included echocardiography, which demonstrated impaired left ventricular function with regional wall motion abnormalities in the hypokinesis (diminished contraction) in the mid and apical segments of the anterior wall (Figure 1). The tricuspid annular plane systolic excursion (TAPSE) was 21 mm, and a slight pericardial effusion was noted. Ultrasound examination revealed significant inferior vena cava (IVC) caliber fluctuation, bilateral pleural sliding, and no free abdominal fluid. The absence of focal neurological deficits and a standard head CT ruled out an embolic stroke [2].
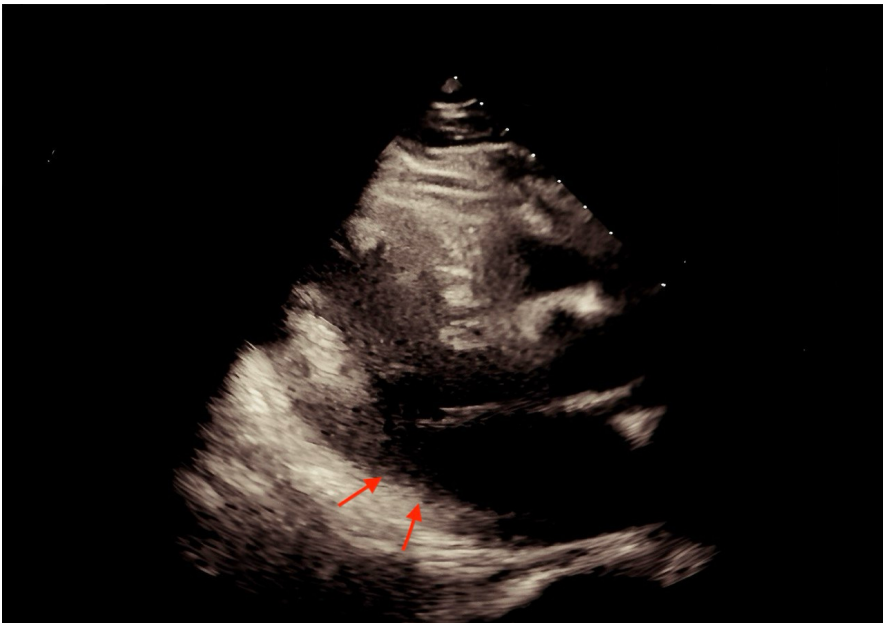 Figure 1: Arrow showing hypokinesis (diminished contraction) in the mid and apical segments of the anterior wall.
Figure 1: Arrow showing hypokinesis (diminished contraction) in the mid and apical segments of the anterior wall.
The patient was managed with intravenous isotonic saline for fluid resuscitation [3], intravenous insulin infusion to normalize blood glucose levels [4], and potassium supplementation for hypokalemia [5]. Continuous cardiac and neurological monitoring was maintained throughout her stay. Her mental status gradually improved over the next 24h, and her blood glucose and osmolality normalized. She was subsequently transferred to the cardiology ward for further management of her AMI and diabetes.
Discussion
While HHS is a well-documented emergency, its occurrence in the early post-percutaneous coronary intervention (PCI) period is relatively underreported. This case contributes to the growing evidence supporting enhanced metabolic surveillance in diabetic patients undergoing cardiac interventions. Further studies should evaluate whether standardized hyperosmolarity screening should be integrated into AMI care protocols, particularly in diabetic patients [6].
This case highlights the onset of HHS in a patient with type 2 diabetes mellitus following urgent coronary intervention for AMI. Fluid resuscitation was critical but delayed due to volume overload concerns, potentially prolonging HHS resolution [7]. The absence of ketones and significant hyperosmolality (365 mOsm/L) supported the diagnosis of HHS rather than diabetic ketoacidosis (DKA) [8]. The metabolic acidosis (pH 7.3, base excess -8 mmol/L) was primarily due to lactic acidosis, likely driven by tissue hypoperfusion. However, underlying renal impairment may have further exacerbated the acid-base imbalance [9]. Sepsis was ruled out due to the absence of fever and negative infectious markers, and stroke was excluded as no focal neurological deficits were observed.
HHS has a reported mortality rate of 10–20%, underscoring its acuity [10, 11]. Early recognition and management are critical to improving outcomes. Management prioritizes IV fluids, insulin, and electrolyte repletion, as outlined in guidelines [12]. In this case, the patient was managed with intravenous isotonic saline, insulin infusion, and potassium supplementation, gradually improving her mental status and metabolic parameters over 24h. This highlights the importance of a multidisciplinary approach involving cardiologists, endocrinologists, and intensivists to optimize glucose control and electrolyte balance in high-risk patients.
Although hyperglycemic crises are common in diabetic patients, their occurrence immediately after AMI intervention is rare. The underlying pathophysiology may involve several mechanisms. First, in our case, AMI likely triggered catecholamine-driven hyperglycemia, exacerbating HHS [10]. Second, medications such as bisoprolol and ramipril, often prescribed post-AMI, may interfere with glucose metabolism and mask early signs of hypovolemia, delaying timely recognition [11]. Third, transient hemodynamic instability following PCI can impair renal function, worsening hyperosmolarity and acidosis [12].
This case underscores the need for routine metabolic surveillance in diabetic patients following AMI. Given the rapid metabolic decompensation observed here, we advocate for protocolized glucose and osmolality monitoring in diabetic post-AMI patients, as supported by existing evidence [1, 5]. Collaboration among cardiologists, endocrinologists, and intensivists is essential to optimize glucose control and electrolyte balance in high-risk patients [13].
Conclusion
HHS is a life-threatening complication of diabetes that can develop following AMI. This case underscores the need for proactive metabolic surveillance in diabetic patients post-AMI to prevent severe complications. Future research should explore the integration of routine metabolic screening into AMI care protocols.
Ethics Approval and Consent to Participate
This case report is exempt from IRB approval as per the policies, as it involves retrospective analysis of anonymized patient data and does not constitute human subjects’ research. Written informed consent was obtained from the patient.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Availability of Data and Materials
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Conflicts of Interest
The author declares no conflict of interest.
Authors' Contributions
Singh A has participated directly in the planning and execution of the work and has approved the final version of the manuscript.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
During the preparation of this work, AI tools (ChatGPT, DeepSeek) were used for grammar and readability improvements. The author reviewed and approved all content, ensuring its accuracy.
Appendices
The Patient Perspective: The patient remained stable during follow-up visits at 3 and 6 months. She adhered to her prescribed medication regimen and demonstrated a positive outlook toward her future health outcomes. By incorporating healthy lifestyle changes and attending more frequent follow-up appointments with her primary care physician, the patient actively contributed to her ongoing recovery and well-being.
References
- Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-343.
- ElSayed NA, Aleppo G, Aroda VR, et al. Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S1-S156.
- Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. 2014;37(11):3124-131.
- Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157-64.
- Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-53.
- Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-21.
- Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-499.
- Umpierrez GE, Khajavi M, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Am J Med Sci. 1996;311(5):225-33.
- Stoner GD. Hyperosmolar hyperglycemic state. Am Fam Physician. 2005;71(9):1723-730.
- Wachtel TJ, Tetu-Mouradjian LM, Goldman DL, et al. Hyperosmolarity and acidosis in diabetes mellitus: a three-year experience in Rhode Island. J Gen Intern Med. 1991;6(6):495-502.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-425.
- Fayfman M, Pasquel FJ, Umpierrez GE. Management of Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Med Clin North Am. 2017;101(3):587-606.
- Kitabchi AE, Umpierrez GE, Fisher JN, et al. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93(5):1541-552.

