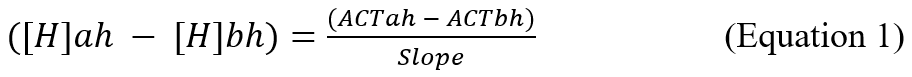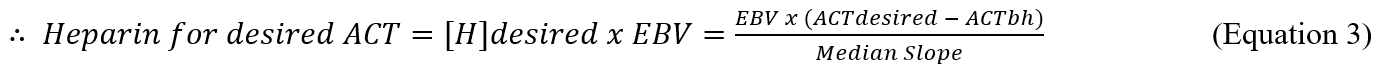Abstract
Introduction: Unfractionated heparin (UFH) has been the standard anticoagulant for cardiac surgery involving cardiopulmonary bypass (CPB) circuits since 1953. However, there is no universally accepted method for determining the most appropriate dose to achieve the desired activated clotting time (ACT) of at least 480 seconds, or an agreed method for determining the appropriate dose and timing of protamine for heparin reversal.
Methods: A technique for determining an initial heparin dose to achieve a desired ACT was developed for catheter ablation cases in patients with atrial fibrillation. This method also enabled the estimation of an appropriate dose of protamine for reversing the active heparin at the end of the procedure. We evaluated this approach in cardiac bypass cases with additional information being provided by the use of viscoelastic testing.
Results: The method used enabled a single dose of heparin to be found that achieved an ACT of more than 480 seconds in over 82% of cases. A single dose of protamine was effective in reversing the heparin effect after cessation of bypass and patient rewarming in 88.89% of cases.
Conclusion: This study presents a promising, cost-effective approach to managing heparin therapy during coronary bypass (CBP) surgery. Our approach enables an appropriate initial heparin dose to be estimated and determination of a dose of protamine that can be given to adequately reverse active heparin at the end of the procedure, after warming and replacement of pump blood.
Keywords
unfractionated heparin, protamine, heparin:ACT dose-response, activated clotting time, cardiac bypass, algorithm, atrial fibrillation, iPhone app
Abbreviations
UFH: unfractionated heparin; CPB: cardiopulmonary bypass; ACT: activated clotting time; CBP: coronary bypass; EBV: estimated blood volume; HMS: hemostasis management system
Introduction
Unfractionated heparin (UFH) has been the standard anticoagulant for cardiac surgery involving cardiopulmonary bypass (CPB) circuits since 1953 [1]. Weight-based algorithms are often used to estimate the initial heparin dose to achieve an activated clotting time (ACT) of more than 480 seconds. The actual dose used varies between institutions, based on tradition, and personal experience. There are several approaches, with a common approach being to administer a dose of 3–5 mg/kg and top up the dose if the ACT is less than 480 seconds, although lower values have been used depending on the circuits employed [2].
There is no consensus on the best method of determining the optimal dose of protamine for the reversal of residual heparin after coronary procedures [3–5].
One of the most common strategies is to estimate the dose using a fixed protamine to heparin ratio based either on the first heparin bolus or the total heparin dose given during coronary bypass (CBP) [6]. Neither approach accounts for heparin administration and metabolism during CPB. Other strategies have been developed to consider heparin metabolism and/or heparin concentration such as statistical or pharmacokinetic modeling of heparin metabolism over time or as a function of baseline and post-heparin ACT. The Hepcon hemostasis management system (HMS) (Medtronic Australasia Pty Ltd, 2 Alma Road, Macquarie Park NSW 2113, North Ryde NSW 1670, Australia) [7] uses a measured heparin concentration to estimate the initial heparin dose and final protamine dose based on a measured heparin concentration, but suffers from a number of problems [8, 9].
These approaches have had limited success in predicting the appropriate dose of heparin to achieve a desired ACT, and the dose of protamine needed to counteract the remaining heparin.
The approach taken in this paper was used in the cardiac catheter laboratory involving patients undergoing catheter ablation for atrial fibrillation as part of an observational study of heparin administration and reversal during catheter ablation for atrial fibrillation [10]. The results have been published in the Journal of Atrial Fibrillation & Electrophysiology [11].
In this paper, 86% of patients achieved a desired ACT of between 300 and 400 seconds after a single dose of heparin, with 92% reversal of heparin to pre-heparin ACT after a single dose of protamine. The approach taken in this study has yet to be validated in cardiac surgery.
The logic behind this approach is as follows (Supplementary, Appendix A):
The volume of distribution of heparin has been shown to be closely approximated to the blood volume [12].
The ‘effective’ heparin concentration can be estimated by dividing the initial heparin dose by the estimated blood volume (EBV) after redistribution of the heparin has occurred.
The initial heparin concentration before heparin is given is considered to be zero.
The slope of the heparin:ACT dose-response curve can be estimated if the ACT is measured before and after the heparin is given.
Knowing the individual heparin:ACT dose-response curve slope enables an estimate of the heparin concentration for a measured ACT.
This allows estimation of subsequent doses of heparin required to maintain a desired ACT, and the protamine dose needed to reverse the active heparin at that ACT.
The median heparin:ACT dose-response curve taken from all patient measurements can be used to estimate the initial heparin dose likely to achieve a desired ACT in future cases.
The objective of this pilot study was to evaluate this approach for initial heparin dose and protamine reversal in the context of cardiac surgery. To this end, the details of cardiac surgery and individual patient medical problems were not considered. The approach taken differs from the approach taken by the Hepcon HMS system in that with this approach the heparin:ACT dose-response curve is based on the pre-heparin ACT, the in vivo ACT measured after a heparin dose is administered to the patient, and estimated heparin concentration found by dividing the heparin dose given by the EBV from the formula of Nadler et al. [13].
This is in contrast with the HMS system, which uses heparin concentrations estimated in a sample of blood taken from the patient. This ignores the heparin-binding to endothelial cells that occurs rapidly after heparin is given. They also use the Allen et al. [14] formula for estimating blood volume, which has been shown to be inferior to the formula derived by Cumpston [15]. The pros and cons of the HMS system have been discussed elsewhere [9, 16].
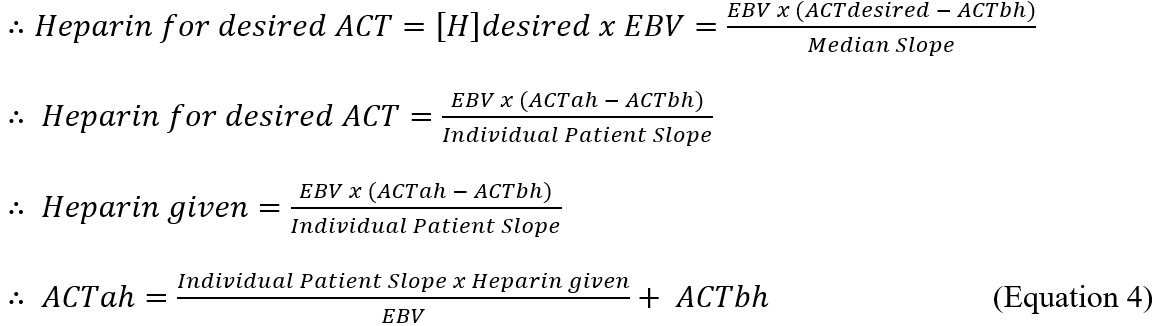
The slope of the HDR curve in our study is calculated using the following formula:

Where:
ACTah is ACT after heparin.
ACTbh is ACT before heparin.
[H]ah is heparin concentration after the initial heparin administration, given by dividing the heparin dose by the EBV using the formula of Nadler et al. [13].
[H]bh is heparin concentration before the initial heparin administration.
If no heparin has been administered beforehand, the [H]bh becomes zero.
Once this relationship has been established, it is possible to estimate the heparin concentration at any ACT. Multiplying the heparin concentration by the EBV gives the total amount of active heparin contributing to that ACT. It is then possible to estimate how much heparin is needed to adjust the ACT to the desired level, and how much protamine to use to reverse that heparin. Further, it is possible to estimate the likely heparin dose to achieve a desired ACT, based on the pre-heparin ACT, the median value for the slope (calculated from all previous patient data), and the EBV as follows:
Equation 1 can be modified as follows:
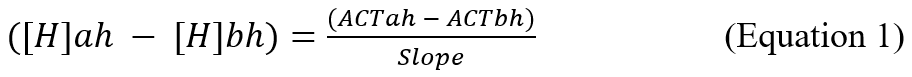
For a new patient:
 given that [H]bh is zero, and using the median slope derived from all patients.
given that [H]bh is zero, and using the median slope derived from all patients.
By interpolation, the desired heparin concentration for the desired ACT is….

Multiplying the estimated heparin concentration by the EBV gives:
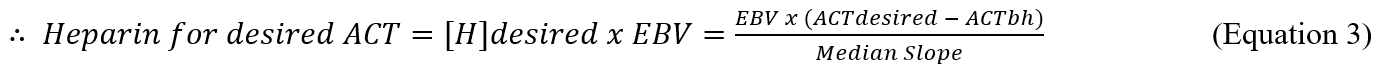
For example, a 70-year-old male patient weighing 97 kg, with a height of 178 cm, a pre-heparin ACT of 120 seconds, and a desired ACT of 600 seconds would need:
Given a median slope of 96.83, and applying equation 2,
Heparin concentration desired = (600 – 120)/96.83 = 4.957
From Nadler et al. [13] formula, the EBV is 5796 mL
Therefore, the heparin dose needed is 5796 × 4.957 = 28,731 IU.
In this real-world example, it was decided to give 30,00 IU. The ACT after this was 610 seconds.
Materials and Methods
This study formed part of a study receiving Ramsay Governance Approval [17]. Patient consent was not required as the data collected formed part of the normal administration of heparin and protamine during CBP procedures. All data was entered into a purpose-written database application, with the SQLite database encrypted. Data was then exported to an anonymized .CSV text file and analyzed using R statistical software [18, 19].
Continuous variables were tested with the Shapiro-Wilk test of normality which showed that the variables being examined showed a non-normal distribution (heteroscedasticity). Those variables that failed to meet the criteria for normal distribution were compared using a two-tailed Wilcoxon rank-sum test with continuity correction.
Robust statistical methods were then used for comparison of variables [20–22].
Differences were considered significant at a level of p < 0.05.
Patient demographics
45 patients were allocated sequentially as part of routine cardiac lists. Their details are shown in the table below (Table 1).
| Variable | Study cohort* (n = 45) |
| Age | 72.31 (56 – 86) |
| Height (cm) | 171.4 (151 – 182) |
| Weight (kg) | 79 (51 – 107) |
| Estimated blood volume1 (mL) | 4915 (3282 – 6092) |
| BMI | 26.91 (19.2 – 44.54) |
| Sex |
| Male | 35 |
| Female | 10 |
Table 1: Patient details expressed as a mean plus range. *Variables are expressed as mean and range. Height, weight, and sex were used to determine an estimated blood volume using the Nadler et al. [13] formula.
Research design
The same anesthetist carried out each procedure. A baseline ACT was taken before heparin administration and an initial heparin dose was estimated, based on the median slope derived from previous experience from the catheter ablation study. After each subsequent patient was entered, the median value for the slope was recalculated. A post-heparin ACT was measured two minutes after the initial dose and the unique HDR slope was derived for that patient. This slope value was then used for further adjustments to the ACT during the procedure. Additional heparin doses were based on heparin concentrations estimated from the ACT and the HDR curve. After the patient was taken off cardiac bypass and rewarmed, a pre-protamine ACT was taken and the amount of active heparin was estimated from the ACT and that patient’s individual HDR curve. Protamine was then given, based on the relationship of 1 mg of protamine being roughly equivalent to 100 IU of heparin. A further ACT was taken three minutes after protamine administration.
The Hemochron Signature Elite (Soma Tech Intl, 166 Highland Park Dr. Bloomfield, CT 06002, USA) was used for all ACT measurements. At the end of the procedure, viscoelastic testing using the TEG 6 device (Haemonetics Corporation, 125 Summer Street Boston, MA 02110, USA) was carried out to determine the presence of ongoing heparin effect, as a normal post-protamine ACT has been shown to be an insufficient indicator of heparin reversal [23].
Results
There were no adverse results related to the use of this approach, and the average on pump time was not affected.
ACT after initial heparin
The range of ACT values after the initial heparin dose is given in the table below (Table 2).
| Minimum | 1st quarter | Median | Mean | 3rd quarter | Maximum |
| 385.0 | 526.0 | 598.0 | 658.1 | 767.0 | 1005.0 |
Table 2: ACT achieved after initial heparin dose.
The ACT desired after the initial dose of heparin was usually set at a target of 600 seconds, but varied according to the patient, with a range of 450–600 seconds. The median value achieved was 598 seconds. The recommendation for bypass procedures is for an ACT of more than 480 seconds [2].
In this study, an ACT of more than 480 seconds was achieved with a single dose of heparin in 37 patients (82.22% of the time) (Figure 1). Knowing the patient’s individual heparin:ACT dose-response curve made it simple to estimate the dose of heparin required to increase the ACT to the desired level.
 Figure 1: Box and whisker plot showing ACT before and after initial heparin dose.
Figure 1: Box and whisker plot showing ACT before and after initial heparin dose.
Given that the anesthetist was using the method being tested as a guide, it was felt wise to check that the dose of heparin given did not differ significantly from the dose estimated. The figure shows no significant difference between the dose of heparin estimated by the method used and the initial dose given (p = 0.378) (Figure 2).
 Figure 2: Box and whisker plot showing no significant difference between heparin predicted and dose given.
Figure 2: Box and whisker plot showing no significant difference between heparin predicted and dose given.
By rearranging Equation 3, we can estimate the ACT that would have been achieved if the dose predicted had been used:
The result of these calculations shows that if the predicted dose of heparin were given, the percentage of cases with an ACT of more than 480 seconds would increase from 82.22% to 95.56%. The range of values is seen in the table below (Table 3).
| Minimum | 1st quarter | Median | Mean | 3rd quarter | Maximum |
| 385.0 | 534.5 | 636.0 | 667.0 | 769.0 | 1005.0 |
Table 3: ACT predicted if the initial heparin dose estimated had been given.
ACT after initial protamine
There was no significant difference between the dose of protamine predicted and that given (p = 0.91) (Figure 3).
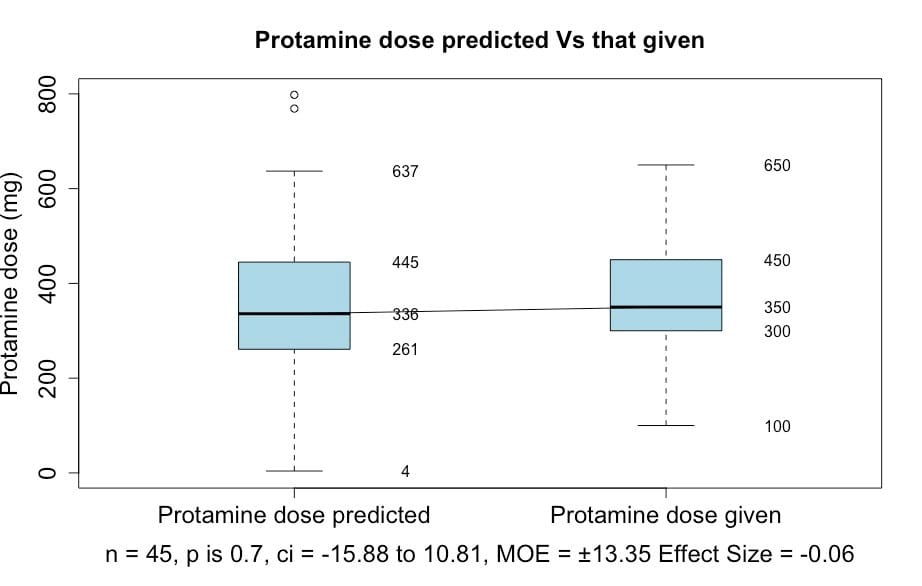 Figure 3: Box and whisker plot showing no significant difference between the estimated protamine dose and that given.
Figure 3: Box and whisker plot showing no significant difference between the estimated protamine dose and that given.
There was a statistically significant difference between the pre-heparin and post-protamine ACT values (p = 0) (Figure 4) but no clinically significant difference. The normal range for ACT is given as either 70–180 seconds or 70–120 seconds, depending on the laboratory. It is generally accepted that an ACT of more than 180 seconds suggests a heparin effect in the context of bypass surgery.
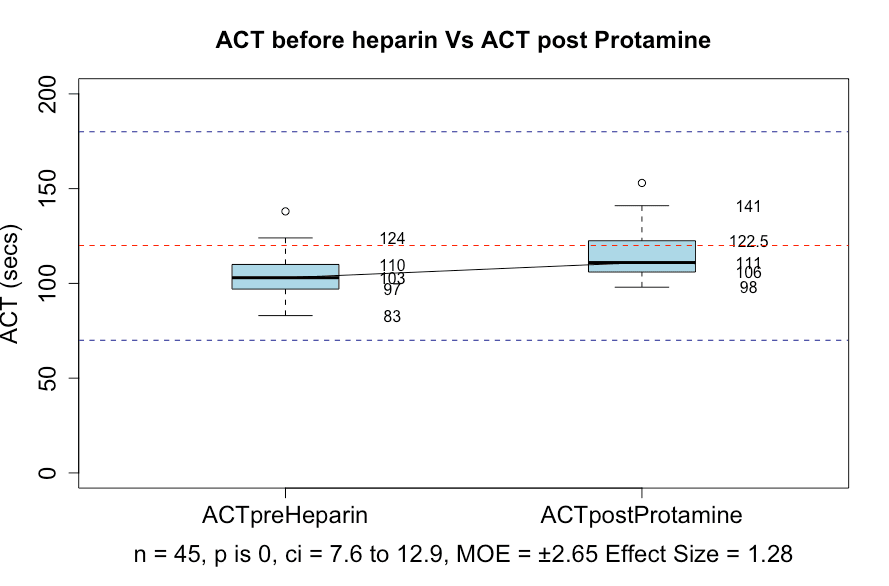 Figure 4: Box and whisker plots showing the ACT before heparin Vs. after protamine was given. Blue dotted lines indicate an ACT of 70 and 180. The red dotted line shows an Act of 120.
Figure 4: Box and whisker plots showing the ACT before heparin Vs. after protamine was given. Blue dotted lines indicate an ACT of 70 and 180. The red dotted line shows an Act of 120.
In this study, post-protamine heparin values were all less than 180 seconds. There were 12 out of 45 cases that had an ACT of more than 120 seconds. Viscoelastic testing with the TEG 6 showed no significant difference between R and R with heparinase, with a p-value of 0.79 (Figure 5). However, 6 patients had evidence of a residual heparin effect as shown by a raised ACT (patients 20, 21, 30, 31, 44, 45). Further investigation showed that patient 20 had been assessed as needing a protamine dose of 798 mg and had been given only 500 mg. That patient had additional protamine and the repeat TEG values indicated complete reversal of heparin. If patient 20 is excluded, then only 5 out of the total of 45 patients (11.11%) had inadequate heparin reversal. That is, 88.89% had a satisfactory reversal of heparin.
Of the remaining patients with a post-protamine ACT of more than 120 seconds, one had a normal TEG with a TEG ACT of 106.6 (patient 21), and two had an identified platelet defect contributing to the increased ACT (patients 6, 28). One had a problem with coagulation factors (patient 14), and one had low fibrinogen and possibly low platelets (patient 15) (Supplementary Appendix B).
It is worth noting that patients 6 and 30 had an ACT of more than 120 seconds before heparin was administered.
This showed that the increase in ACT over 120 seconds for these remaining five cases was contributed to by factors other than inadequate heparin reversal.
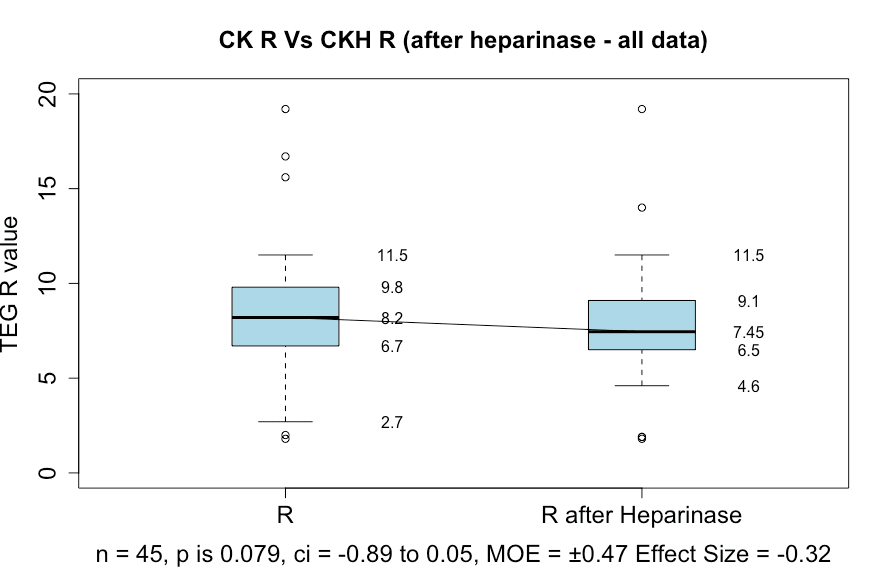 Figure 5: Box and whisker plots showing the TEG 6 R-value before and after heparinase.
Figure 5: Box and whisker plots showing the TEG 6 R-value before and after heparinase.
Discussion
This work shows that the creation of an individual patient’s heparin:ACT dose-response curve can be used to estimate the dose of protamine needed to reverse heparin at the end of CBP procedures with a success rate in the order of 89%. The use of viscoelastic testing helped elucidate the cases where heparin or other factors were causing an ACT of greater than 120 seconds. When the median slope for the HDR curves of all cases was used to predetermine a likely heparin dose to achieve a value of more than 480 seconds, a success rate of 82% was achieved. In those cases where the ACT proved to be less than desired, the individual patient’s HDR curve could be used to determine an appropriate dose of heparin to achieve the desired result.
This approach is considerably cheaper and simpler to implement than using the Hepcon HMS device, with its described problems. It can be used with any ACT device that has a linear response between heparin concentration and measured ACT.
The significance of this work is that it provides a straightforward method for determining the initial heparin dose and takes a lot of the guesswork out of estimating an appropriate protamine dose for heparin reversal after coming off bypass and rewarming the patient. It also eliminates the need for a protamine test dose, with its consequent risks [5].
Limitations
This approach depends on a number of assumptions. The blood volume estimation depends on work done in adults and may not be as accurate in children. Further work needs to be done to improve algorithms for estimating blood volume over a range of ages and body types. The assumption that the heparin:ACT dose-response curve is linear over the range seen in cardiac surgery may not be correct. Dilution of the blood during bypass leads to reduced heparin concentration as measured by anti-Xa levels, while the ACT remains high. However, the amount of heparin and the amount of blood components it is binding to remain relatively unchanged (except in the case of major blood loss). It may be that even on bypass, the HDR curve can be used to adjust heparin dosing, but this has not been tested in this study. After coming off the bypass and rewarming the patient, the HDR curve can be used to determine the appropriate protamine dose, as has been shown in this paper. Changes in the actual blood volume and its components may have accounted for some of the cases where the post-protamine ACT was more than 120 seconds.
This paper addresses only the management of heparin and protamine and does not address other causes of perioperative bleeding and its treatment. There were no adverse results related to the use of this approach, and the average on pump time was not affected.
Conclusions
This study presents a promising, cost-effective approach to managing heparin therapy during CBP surgery.
Creating a patient-specific heparin:ACT dose-response curve allows for a more tailored initial heparin dose compared to a standard approach. This achieved an 82% success rate in reaching the desired ACT level of more than 480 seconds.
A recent paper by Crivellari et al. [24] summarizes the interactions between protamine and heparin. They state that historically, protamine has been given at a 1:1 ratio relative to the initial or even total dose of heparin previously administered. They point out that this ratio may lead to excessive bleeding due to the anticoagulant properties of protamine. They remain, however, uncertain as to the optimal strategy for heparin antagonism.
Despotis et al. [25] have demonstrated that a reduction in the protamine‑to‑heparin ratio decreased postoperative bleeding and the amount of fresh‑frozen plasma and platelet administration after CPB. Miles et al. [4] used a mathematical model to guide protamine dosing in patients following CPB with improved TEG R-time and reduced the dose administered relative to a fixed ratio. No differences were detected in postoperative mediastinal/pleural drainage or red blood cell transfusion requirement in their cohort of low-risk patients.
The use of the individual patient’s HDR curve allows estimation of the protamine dose needed to reverse heparinization after bypass and rewarming, with an 89% success rate. This reduces reliance on guesswork and potentially avoids complications associated with excess protamine.
This method utilizes readily available ACT devices and avoids the complexities and costs associated with the Hepcon Heparin Management System.
Areas for future research are indicated as follows:
- Blood volume estimation needs further research, particularly for children.
- The linearity of the HDR curve during bypass requires validation.
- HDR curve adjustments/alterations during bypass itself needs investigation.
Author Contributions
Dr. Cumpston wrote the software required for data collection and estimating heparin and protamine doses, analyzed the data, generated the images, and wrote the first draft of the manuscript. Dr. Duffy provided anesthetic services for all cardiac patients, collected the data to be analyzed, including the TEG data, and reviewed the original manuscript, providing suggestions for alterations and editions. Both authors were involved in reviewing the revised draft of the manuscript.
Conflicts of Interest
The principal author has written a number of computer applications incorporating the methodology used in this paper. One such application is available on the iPhone App Store as ‘Heparin Heuristics Lite’ (https://apps.apple.com/us/app/heparin-heuristics-lite/id1499772855).
Funding
No funding was obtained for this research.
Supplementary Material
References
- Cohn LH. Fifty years of open-heart surgery. Circulation. 2003;107(17):2168-170.
- Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg. 2020;57(2):210-51.
- Levy JH, Ghadimi K, Kizhakkedathu JN, et al. What’s fishy about protamine? Clinical use, adverse reactions, and potential alternatives. J Thromb Haemost. 2023;21(7):1714-723.
- Miles LF, Burt C, Arrowsmith J, et al. Optimal protamine dosing after cardiopulmonary bypass: The PRODOSE adaptive randomised controlled trial. PLoS Med. 2021;18(6):e1003658.
- Jansa L, Fischer C, Serrick C, et al. Protamine Test Dose: Impact on Activated Clotting Time and Circuit Integrity. Ann Thorac Surg. 2022;113(2):506-10.
- Codispoti M, Ludlam CA, Simpson D, et al. Individualized heparin and protamine management in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71(3):922-27.
- Erdoes G, Koster A. Heparin/Protamine Management With the Hepcon HMS: Is There More to Consider Than Printed Values? J Cardiothorac Vasc Anesth. 2019;33(8):2161-162.
- Garvin S, FitzGerald DC, Despotis G, et al. Heparin concentration-based anticoagulation for cardiac surgery fails to reliably predict heparin bolus dose requirements. Anesth Analg. 2010;111(4):849-55
- Gilly G, Trusheim J. Con: The Hepcon HMS Should Not Be Used Instead of Traditional Activated Clotting Time to Dose Heparin and Protamine for Cardiac Surgery Requiring Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2016;30(6):1730-732.
- “An observational study of heparin administration and reversal during catheter ablation for atrial fibrillation (CA-AF).” (UTN: U1111-1219-6648).
- Cumpston P, Phillips PK. A Novel Method for Calculating Heparin Dosing to Rapidly Achieve Target Anticoagulation for Cardiac Procedures. Journal of Atrial Fibrillation & Electrophysiology (JAFIB-EP). 2024;17(2):p29.
- Estes JW. Clinical pharmacokinetics of heparin. Clin Pharmacokinet. 1980 May-Jun;5(3):204-20.
- Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-32.
- Allen TH, Peng MT, Chen KP, et al. Prediction of blood volume and adiposity in man from body weight and cube of height. Metabolism. 1956;5(3):328-45.
- Cumpston P. Blood volume estimation in cardiac surgery – A comparative analysis. Perfusion. 2023;38(3):455-63.
- Ural K, Owen C. Pro: The Hepcon HMS Should Be Used Instead of Traditional Activated Clotting Time (ACT) to Dose Heparin and Protamine for Cardiac Surgery Requiring Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2016;30(6):1727-729.
- Ramsay Governance.”Validation of a method for heparin reversal with protamine in cardiac surgery. An observational cohort study of patients undergoing cardiac surgery.” Approved 30 August 2021 (Ref: 21/08).
- The R Foundation for Statistical Computing, Vienna, Austria.
- Wilcox RR. Understanding and Applying Basic Statistical Methods Using R. USA: Wiley; 2016.
- Erceg-Hurn DM, Mirosevich VM. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. Am Psychol. 2008;63(7):591-601.
- Mair P, Wilcox R. Robust statistical methods in R using the WRS2 package. Behav Res Methods. 2020;52(2):464-88.
- Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing: USA: Elsevier; 2016.
- Maslow A, Chambers A, Cheves T, et al. Assessment of Heparin Anticoagulation Measured Using i-STAT and Hemochron Activated Clotting Time. J Cardiothorac Vasc Anesth. 2018;32(4):1603-608.
- Crivellari M, Landoni G, D’Andria Ursoleo J, et al. Protamine and Heparin Interactions: A Narrative Review. Ann Card Anaesth. 2024;27(3):202-12.
- Despotis GJ, Joist JH, Hogue CW Jr, et al. The impact of heparin concentration and activated clotting time monitoring on blood conservation. A prospective, randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;110(1):46-54.