Abstract
Calcium channel blocker (CCB)-induced vasoplegia syndrome is an emergent, life-threatening complication that can occur refractory to traditional therapy in the treatment of an acute CCB overdose. We present a case of acute co-ingestion of many prescription medications, including amlodipine and escitalopram. The patient presented with hemodynamic instability that persisted despite conventional therapies. Hydroxycobalamin (HCO) therapy was initiated, resulting in hemodynamic and clinical improvement. We highlight the unique presentation of this case and the potential benefits associated with HCO administration.
Key Points: This case demonstrates the potential benefits of HCO administration in CCB-induced vasoplegia syndrome. Given the significant morbidity and mortality associated with CCB toxicity, HCO may provide considerable benefit in patients with hemodynamic instability refractory to standard treatment modalities.
Keywords
hydroxycobalamin, vasoplegia, hypotension, mean arterial pressure, overdose
Abbreviations
HCO: hydroxycobalamin; MAP: mean arterial pressure; CCB: calcium channel blocker; VA-ECMO: venoarterial extracorporeal membrane oxygenation; SSRIs: selective serotonin reuptake inhibitors; HDIET: high-dose insulin euglycemic therapy
Patient Case
A 34-year-old female with a past medical history significant for bipolar disorder, hypertension, hypothyroidism, and substance abuse presented to the emergency department following an intentional overdose of prescribed medications. The patient claimed to have taken a large amount of several home medications; however, she was unclear as to which agents and specific quantities were consumed. The patient claimed to have consumed several alcoholic energy drinks within the same time period. The previously prescribed home medications are listed in the table (Table 1).
| Home medications |
| Amlodipine 10 mg tablet once daily |
| Escitalopram 10 mg tablet once daily |
| Levothyroxine 25 mcg tablet once daily |
| Oxcarbazepine 300 mg tablet once daily |
| Pravastatin 40 mg tablet once daily |
| Quetiapine 50 mg tablet once daily at bedtime |
Table 1: Reported list of previously prescribed home medications. Abbreviations – mcg: micrograms; mg: milligrams.
On initial evaluation, the patient was markedly hypotensive with a blood pressure of 64/48 mmHg, mean arterial pressure (MAP) 53 mmHg, pulse 109 beats/min, respiratory rate 20 breaths/min, pulse oximetry 96% on room air, and afebrile. The patient was notably intoxicated with no focal findings on the physical exam. The patient denied any acute distress, pain, shortness of breath, nausea, diaphoresis, or dizziness.
Initial electrocardiogram findings demonstrated sinus tachycardia without axis deviation. Chest X-ray showed an indiscriminate pulmonary interstitial prominence representing potential atypical or viral pneumonia or edema. Laboratory studies demonstrated hypokalemia with potassium of 2.6 mEq/L, serum ethanol of 244 mg/dL, and lactic acidosis with lactate of 3.38 mmol/L and pH of 7.27. Urine toxicology, acetaminophen level, and salicylate level showed no abnormalities.
As the patient presented with significant hemodynamic instability, management was directed toward a probable overdose of a calcium channel-blocking agent. Treatment was initiated with one liter of 0.9% sodium chloride intravenous fluid bolus, 1000 mg calcium gluconate IVP, potassium chloride 40 mEq IV, and a norepinephrine infusion. A central line was placed for vasoactive medication administration. Due to progressive hypoxemic respiratory distress, the patient was intubated and transferred to the intensive care unit. Following the transfer, an arterial line was placed for continuous hemodynamic monitoring.
After four liters of 0.9% sodium chloride and three additional calcium gluconate boluses, the patient remained hypotensive with a blood pressure of 73/36 mmHg and MAP of 48 mmHg. Over the next 12 h, vasopressin, phenylephrine, and dobutamine infusions were initiated in addition to high-dose insulin euglycemic therapy (HDIET). As the patient was significantly acidotic, multiple sodium bicarbonate 50 mEq intravenous boluses were administered, followed by a continuous IV infusion. The patient remained hemodynamically unstable with a blood pressure of 59/56 mmHg, MAP of 57 mmHg, and worsening serum lactate of 13 mmol/L. The multidisciplinary team did not utilize IV lipid emulsion therapy as the patient was receiving propofol for sedation.
Toxicology was consulted and recommended treatment with methylene blue. Given the potential for concomitant escitalopram overdose, however, treatment with hydroxycobalamin (HCO) was agreed upon by the multidisciplinary team. HCO was administered at a dose of 5 g over 15 min. Following administration, significant improvements in hemodynamic stability were observed with a blood pressure of 101/53 mmHg and MAP 69 mmHg. Repeat laboratory tests revealed a lactate of 6.2 mmol/L and a pH of 7.3.
Within 24 h of HCO administration, all previously discussed drug therapy had been weaned or discontinued. The patient remained on two vasopressors which were actively being titrated off. Laboratory and hemodynamic markers continued to improve, and the patient was extubated. On day eight of admission, the patient was transferred to a behavioral health unit.
Discussion
This case report highlights the potential role of HCO in the treatment of persistent hemodynamic instability secondary to calcium channel blocker (CCB) overdose refractory to standard pharmacotherapy. This case is the first documented evidence of HCO use for this indication at the time of publication.
CCBs like amlodipine, inhibit L-type voltage-gated calcium channels. The resulting inhibition of calcium influx into smooth muscle cells prevents calcium-dependent myocyte contraction. As the vasculature is the main site of action for dihydropyridine CCBs, toxic ingestion can lead to potent vasodilation and subsequent hypotension [1]. The onset of antihypertensive effects of amlodipine can typically be observed 24–48 h after one dose or earlier in the case of toxic ingestion [2]. The reported time to peak plasma concentration of amlodipine is variable and ranges from 6–12 h, and half-life elimination ranges from 30–50 h. These pharmacokinetic qualities contribute to the significant morbidity and mortality seen with toxic CCB ingestions. After ingestion, hypotension can progress rapidly and remain persistent for several days, as seen in our patient.
Recommendations for the management of acute CCB overdose were recently published in an expert consensus in 2017 [3]. Intravenous fluid resuscitation, calcium administration, vasopressor/inotropic therapy, and HDIET are among first-line recommendations. In the case of myocardial dysfunction, high-dose, incremental doses of insulin and lipid emulsion therapy can be considered. If hemodynamic instability persists, venoarterial extracorporeal membrane oxygenation (VA-ECMO) can be considered for rescue therapy [3].
Evidence exists supporting the potential utilization of methylene blue as an additional pharmacotherapeutic intervention prior to consideration of VA-ECMO. A recent publication presented a patient case with positive outcomes following methylene blue administration. The authors compared the case with ten previously published case reports and one animal study in which improvements in pulse rate, MAP, survival time, and systemic vascular resistance were observed after methylene blue administration [4]. In our institution, methylene blue has been utilized in the management of vasoplegia syndrome following cardiothoracic surgery with supporting evidence [5, 6]. Vasoplegia is a clinical syndrome characterized by the overproduction of nitric oxide, leading to profound vasodilation and subsequent hemodynamic instability refractory to conventional vasopressor therapy. Nitric oxide stimulates soluble guanylyl cyclase resulting in endothelial smooth muscle relaxation. Methylene blue inhibits nitric oxide synthase resulting in improvements in systemic vascular resistance. Given the potential vasoplegic nature of our patient, methylene blue was suggested as a potential treatment. Due to the probable toxic co-ingestion of escitalopram, however, the multidisciplinary team excluded methylene blue utilization due to the risk of serotonin toxicity. Following the exclusion of methylene blue for this patient, HCO was evaluated as a potential alternative at the recommendation of the clinical pharmacist on the team.
The utilization of HCO has been suggested as an alternative to methylene blue in the treatment of vasoplegia syndrome with supporting evidence [7]. HCO acts as a nitric oxide scavenger resulting in decreased stimulation of guanylyl cyclase. Administration in the setting of refractory vasoplegia has been associated with clinically significant increases in blood pressure and subsequent hemodynamic stability [8–10]. These findings are further supported by our patient’s case. Prior to HCO administration, MAPs were consistently within 45–60 mmHg. Following HCO administration, our patient experienced significant improvements in systolic blood pressure and MAP, as seen in the figure (Figure 1).
No adverse effects were observed in this patient’s case. Because methylene blue can interact with selective serotonin reuptake inhibitors (SSRIs) leading to an increased risk of serotonin toxicity, this interaction is avoided when using HCO as an alternative agent. Of note, there have been reports of skin discoloration leading to photosensitivity and renal dysfunction secondary to calcium oxalate crystal formation with HCO administration [11].
The positive outcomes observed in our patient are significant, as no reports exist supporting the use of HCO for CCB-induced vasoplegia. However, the clinical applicability of this case may be somewhat limited due to the co-ingestion of other pharmacotherapeutic agents reported by the patient. In a case reported by Ahmed et al., the authors discussed the potential attribution of the return of hemodynamic stability to the development of serotonin syndrome [4]. Although the interaction between SSRIs and methylene blue was avoided in our patient, serotonin syndrome remains a consideration, given the unknown quantity of escitalopram ingested. Furthermore, hemodynamic stability may have been attributable to recommended therapies such as calcium, vasoactive agents, and HDIET. However, given the lack of hemodynamic response seen prior to HCO administration, this correlation remains unlikely.
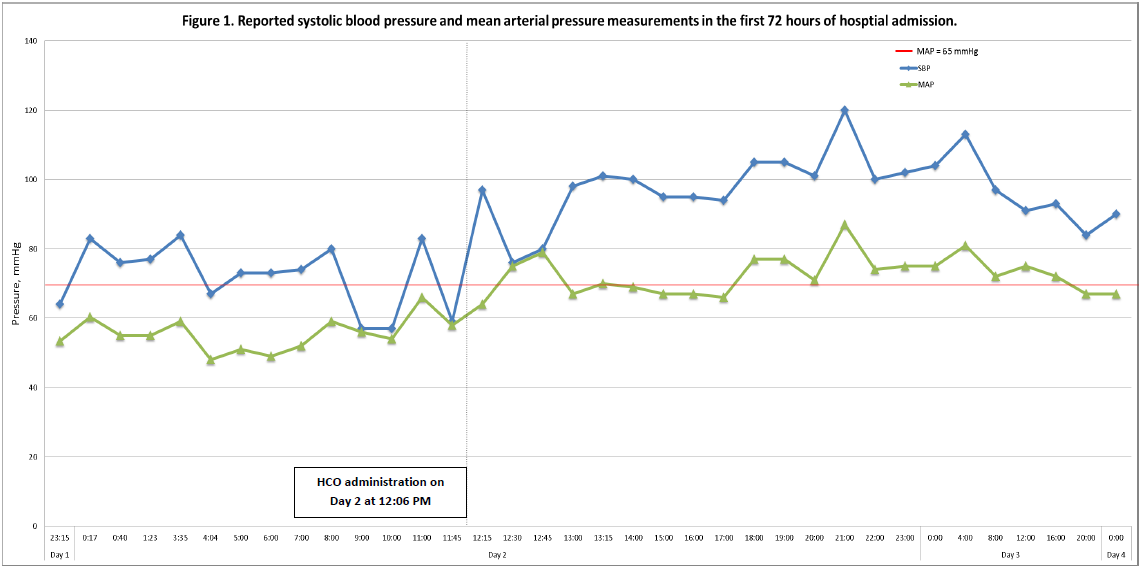 Figure 1: Reported systolic blood pressure and mean arterial pressure measurements in the first 72 h of hospital admission. Hydroxycobalamin administration is indicated by the vertical dashed line at 12:06 pm on day 2. Abbreviations – HCO: hydroxycobalamin; SBP: systolic blood pressure; MAP: mean arterial pressure.
Figure 1: Reported systolic blood pressure and mean arterial pressure measurements in the first 72 h of hospital admission. Hydroxycobalamin administration is indicated by the vertical dashed line at 12:06 pm on day 2. Abbreviations – HCO: hydroxycobalamin; SBP: systolic blood pressure; MAP: mean arterial pressure.
Conclusion
This case demonstrates the potential benefits of HCO administration in CCB-induced vasoplegia syndrome. Currently, there have been no reports detailing the clinical utility of HCO for this indication. Further studies are necessary to support our findings. However, given the significant morbidity and mortality associated with CCB toxicity, HCO may provide considerable benefit in patients with hemodynamic instability refractory to standard treatment modalities.
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Author Contributions
RMC: Concept and design, critical writing of intellectual content, and final approval of the version to be published. SRB: Direct involvement in the patient case at presentation, revising the intellectual content, and final approval of the version to be published. BW: Direct involvement in the patient case at presentation, revising the intellectual content, and final approval of the version to be published.
References
- Hofer CA, Smith JK, Tenholder MF. Verapamil intoxication: a literature review of overdoses and discussion of therapeutic options. Am J Med. 1993;95(4):431-38.
- McAllister RG Jr, Hamann SR, Blouin RA. Pharmacokinetics of calcium-entry blockers. Am J Cardiol. 1985;55(3):30B-40B.
- St-Onge M, Anseeuw K, Cantrell FL, et al. Experts Consensus Recommendations for the Management of Calcium Channel Blocker Poisoning in Adults. Crit Care Med. 2017;45(3):e306-e315.
- Ahmed S, Barnes S. Hemodynamic improvement using methylene blue after calcium channel blocker overdose. World J Emerg Med. 2019;10(1):55-58.
- Mehaffey JH, Johnston LE, Hawkins RB, et al. Methylene Blue for Vasoplegic Syndrome After Cardiac Operation: Early Administration Improves Survival. Ann Thorac Surg. 2017;104(1):36-41.
- Omar S, Zedan A, Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci. 2015;349(1):80-88.
- Ortoleva JP, Cobey FC. A Systematic Approach to the Treatment of Vasoplegia Based on Recent Advances in Pharmacotherapy. J Cardiothorac Vasc Anesth. 2019;33(5):1310-314.
- Roderique JD, VanDyck K, Holman B, et al. The use of high-dose hydroxocobalamin for vasoplegic syndrome. Ann Thorac Surg. 2014;97(5):1785-786.
- Shah PR, Reynolds PS, Pal N, et al. Hydroxocobalamin for the treatment of cardiac surgery-associated vasoplegia: a case series. Can J Anaesth. 2018;65(5):560-68.
- An SS, Henson CP, Freundlich RE, et al. Case report of high-dose hydroxocobalamin in the treatment of vasoplegic syndrome during liver transplantation. Am J Transplant. 2018;18(6):1552-555.
- Hydroxocobalamin injection [prescribing information]. Parsippany, NJ: Actavis Pharma, Inc.; 2018.
![]() *, Baty SR and Williams B
*, Baty SR and Williams B
