There are many evidence that links cardiovascular (CV) complications to primary hyperparathyroidism (PHPT). However, these CV complications have never been considered an indication for surgical management of PHPT, and even some studies showed that in those that have surgical treatment, there is no evidence of reversibility of the CV complications. Despite the reasonable explanation of the underlined mechanisms involved in these complications in the literature that prove the increased CV associations, there are some conflicting data that showed no or even reduced associations and this has been a matter of debate for decades. The CV complications reported to have associations with PHPT include cardiac (left ventricular remodeling, coronary vascular disease, calcifications), vascular including hypertension (HTN), atherosclerosis, cardiometabolic complications including insulin resistance, diabetes mellitus, and CV mortality. This article reviewed the strength of current evidence and the author’s stand on whether it is worth including in the guideline as an indication for current treatment.
Primary hyperparathyroidism (PHPT) is the commonest cause of hypercalcemia in an outpatient setting and is the third most common endocrine disorder affecting 0.3% of the general population [1]. The biochemical and clinical phenotype of PHPT has been noticed to be changing in the last few decades. The implicated factor is said to be due to the advent of a multichannel analyzer in 1870 making it to be an incidental asymptomatic disorder rather than the florid disease it used to be [1]. The known classical symptoms of PHPT are the skeletal and renal manifestations [2]. Studies, however, have shown that it is associated with some unusual characteristics which are not routinely assessed in clinical settings, especially cardiovascular (CV) and neuropsychiatric features, both of which have conflicting evidence in the literature.
There is a paucity of CV complications in the literature, although the relationship between PHPT and CV risks has been reported over the past 70 years [3]. The research looked at various aspects of CV risk. Various studies have discussed mechanisms relating to these CV complications, which include cardiac-related problems such as cardiac arrhythmias, left ventricular (LV) dysfunctions, and cardiac calcification. Some studies also discussed an increased risk of vascular and cardiometabolic complications which include hypertension (HTN), atherosclerosis, insulin resistance and diabetes mellitus, metabolic syndrome (MS), and CV deaths in patients with PHPT [4, 5].
Currently, many of the studies available are observational and inconsistent, and the evidence around parathyroidectomy outcomes in terms of CV effects is conflicting. This creates a challenge in concluding the significance in the treatment of patients with PHPT from a cardiovascular perspective. The different presentations, as well as various types of PHPT, make it difficult to streamline one approach to management. The question is, “Should we include a CV examination and investigation screen in the workup of patients with PHPT as part of the complication screen, and in which patients should we offer parathyroidectomy solely based on the CV symptoms, and are there data to suggest reversibility after the definitive treatment of PHPT?
The aim of this review is to critically analyze the strength of current evidence on the significance of cardiovascular complications in patients with PHPT as an indication for any intervention and discuss the controversial evidence.
Cardiac complications of PHPT
The heart is one of the various organs in which the parathyroid hormone has paracrine or autocrine roles. It has been demonstrated by several studies that there is an increased prevalence of structural heart disease as well as functional abnormalities in patients with PHPT; however, several studies show a contrary opinion.
Several studies support an association of LV hypertrophy with PTH level in PHPT because excess PTH leads to the accumulation of calcium in the cells, where the ion triggers protein kinase C activity and, hence, induces hypertrophy [6]. Both in-vivo and in-vitro studies demonstrated this high prevalence of LV hypertrophy in patients with PHPT and regression after parathyroidectomy takes many years though eventually completed [7]. In a study by Piovesan et al., there is a regression in LV hypertrophy after surgery, however, other studies showed contrary outcomes [8]. The functional problems especially LV diastolic contradict this finding. In a case-control study by Dalberg et al., the E/A ratio was found to be lower in patients with PHPT compared with the control [9]. Näppi et al. also found prolonged isovolumetric relaxation time which is indicative of diastolic filling impairments [10]. Although, the methodology of all these studies has been questionable, and the interpretation should be drawn cautiously.
Increased risks of dystrophic calcifications of the valves and the myocardium have been demonstrated by multiples studies especially affecting the left-sided heart valve annuli and cusps. In a prospective study by Stefenelli et al., 63% of the included PHPT patients had calcifications of the aortic valve (50% mild) and 49% of the mitral valve, compared to 12.5% and 15%, respectively, among sex and age-matched controls [4]. In the majority of cases, these alterations were associated with myocardial calcifications. The electrocardiogram abnormalities include QT interval shortening, sometimes prolonged PR interval, and QRS duration are associated with hypercalcemia which is an indication for definitive treatment on its own. A short QT is associated with an increased risk of arrhythmias and sudden cardiac death [11, 12]. Pepe et al. demonstrated that the significantly increased prevalence of supraventricular and ventricular arrhythmia is reduced post-parathyroidectomy [11].
Vascular complications of PHPT
HTN and atherosclerosis are the main vascular complications known to be associated with PHPT and the implicated factors are both hypercalcemia as well a calcium independent PTH pathways. The evidence for its association and the effect of parathyroidectomy on the reversal of these complications are conflicting. Schiffl et al. showed the prevalence of systemic HTN in PHPT is 40–50% [13]. However, increased PTH levels is also associated with primary HTN in patient without PHPT. Thus, it is difficult to correct for the normally elevated PTH in association with primary HTN rather than the contribution from PHPT. In his case-control observational study, Nainby-Luxmoore et al. clearly demonstrated the high prevalence of HTN in hyper parathyroid patients after excluding the confounding factors [14]. However, in a clinical trial by Ringe, 20 out of 27 hypertensive patients with systemic HTN prior to surgery had a reversal of their systemic HTN after parathyroidectomy [15].
Various observational studies demonstrated multiple factors involved in the mechanisms of HTN in these patients which include hypercalcemia, PTH, hyperaldosteronism, and hypomagnesemia with their effects on both peripheral resistance and cardiac output. The proposed mechanisms contributing to increased peripheral resistance in PHPT hypertension include abnormal a aldosterone-to-renin ratio (ARR), dysfunctional changes in vessel walls either due to altered vasodilatory response, diabetes, dyslipidemia, or due to increased reactivity of the CV system to pressor hormones, increased cytosolic calcium in vascular smooth muscle cells resulting in increased vascular tone, vascular reactivity to vasopressors, and increased peripheral vascular tone [16]. Schiffl et al. showed that HTN in hyperparathyroidism is due to the functional alteration of smooth muscle cells in vessels by the direct effect of calcium, and parathyroidectomy can only reverse this situation if the patient does not already have primary HTN [13]. In a literature review by Campese, it was shown that PTH at pharmacological doses antagonises the effects of NE and Ang II but in the same study, it was shown that PTH also increases the pressor effects of hypercalcemia [17]. This was similar to the finding of the clinical control trial by Christensson et al. [18]. Verheyen et al. studied the role of ARR and FGF-23 in causing HTN in PHPT patients and demonstrated that ARR is directly related to high blood pressure in PHPT, and hence proved the association of high aldosterone and prevalence of HTN in PHPT [19]. Hypomagnesemia which is also related to the level of calcium has also been associated with the pathogenesis of HTN seen in PHPT [20].
The evidence of atherosclerosis is also controversial though some studies illustrated a strong point for its increase in PHPT while others showed no relationship. PTH is involved in the expression of the receptor for advanced glycation end products (RAGE) and interleukin 6 which increases endothelial atherosclerosis, increases the production of endothelial nitric oxide synthase which in turn increases NO production [21], increases VEGF 165 mRNA expression and promotes vascular growth and atherosclerosis [22], exerts a direct vasodilatory effect by inhibiting calcium channels in smooth muscle cells [23]. The atherosclerotic complication mechanisms are relating to the direct effects of PHPT rather than hypercalcemia as Nelson et al. demonstrated. [24]. On the other hand, Baykan et al. demonstrated that FMD of the brachial artery was negatively related to serum calcium level [25]. Studies show that there is an impairment of flow-mediated dilation (FMD) of the brachial artery in PHPT, and this marker is considered the gold standard for atherosclerosis. There were improvements of these parameters after parathyroidectomy [24]. One study also showed no improvement in FMD even after 3 years post-parathyroidectomy [26]. Tuna et al. study showed an impairment of FMD in mild PHPT, improving after parathyroidectomy, but did not prove its association with calcium and PTH [27]. Carrelli et al. showed in their study that in mild PHPT, patients have abnormal FMD that remains unchanged after 1-year post parathyroidectomy [28]. Baseline abnormal FMD may normalize after surgery, but he did not support that endothelial dysfunction can be used as an indicator for parathyroidectomy. Therefore, it is difficult to conclude that PHPT is the factor solely responsible for all the endothelial dysfunction [29, 30].
Cardiometabolic complications in PHPT
Cardiometabolic complications are also common including metabolic syndrome, insulin resistance in PHPT and are not also without their conflicting evidence. The association between PHPT and metabolic syndrome (MS) also showed inconclusive evidence. In a retrospective case-control study comparing the CV risk factors between 363 cases of PHPT and 363 controls in New Jersey, USA, there is a higher prevalence of metabolic disorders in patients with PHPT [31]. This is similar in finding to another study comparing 139 cases of PHPT to 111 matched controls which showed that patients with severe PHPT have an increased probability of MS with odds ratio (OR) of 3.5 (95% confidence interval (CI) 1.5–8.125, p = 0.004) and insulin resistance (OR: 3.7, 95% CI 1.64–8.29, p = 0.002) and that serum calcium is a predictor of these CV risk factors [32]. However, in a case-control study by Tassone et al., there is no difference in the risk of MS between the PHPT and the non-PHPT groups [33].
Insulin resistance and T2DM could also complicate PHPT and both the calcium and PTH levels have been suggested to contribute to the mechanism. The data regarding the association of insulin resistance in PHPT are inadequate and contradictory. This is measured by insulin-like growth factor-binding protein-1 (IGFBP-1), which modulates the activity of IGF and its level is inversely proportional to the level of insulin resistance, HTN, and other CV risk factors. The sustained reduction in glucose, insulin, and IGF-1 and the increase in IGFBP-1 after parathyroid surgery strengthen the possible reversibility of the MS coupled with PHPT [32].
Increased mortality in symptomatic PHPT have been associated with high prevalence of the other cardiovascular complications described previously due to higher incidence of HTN, arrhythmias, LV hypertrophy, heart failure, and calcific disease. One mortality study looked at 4,461 patients who underwent parathyroidectomy between 1987 and 1994. The study found PHPT to have an increased risk ratio of 1.71 in men and 1.85 in women for both all-cause mortality and CV death [7]. Evidence showed that early treatment of parathyroid disorders through medical or surgical management may reverse CV remodeling and mitigate cardiac risk factors [34]. However, in a large patient series national registry based observational study assessing the mortality risk post-parathyroidectomy from cardiovascular cause with risk ratios for death being 1.71 for male and 1.85 for female patients (n = 4461), with 95% confidence limits of 1.34–2.15 and 1.62–2.11 for men and women, respectively [35].
Reversibility of cardiovascular complications with PHPT Treatment
The aim of PHPT treatment include normalization of serum calcium and PTH levels and improving the symptoms of the disease and improve the end organ damage [36]. Surgery is the consensus for a definite cure for PHPT and the cure rate of 95–99% in patients with good pre-op localization [37, 38]. Surgery has been found to be more effective and cost-effective than conservative treatment but not all patients would be offered surgery due to its invasiveness and lack of evidence of its benefit especially in the recent biochemical variant of PHPT [39]. The guidelines for surgical management of PHPT are based on age, end-organ disease, and symptomatic hypercalcemia [40].
Currently, recommendations by professional endocrine and surgical societies do not include CV risk factors and complication for routine screening and also as an indication for parathyroid surgery [24]. The currently available data for association between PHPT and CV risk factors are inadequate and controversial as outlined above and also successful parathyroid surgery does not consistently lead to improvement even in the hypercalcemic PHPT [24]. It has been thought that there is no change to CV risks and complications post- parathyroidectomy as PHPT has caused enduring damage to the CV system prior to any treatment [41] and the currently available data showed no conclusive evidence regarding potential CV benefit from parathyroidectomy [37]. Some studies, however, showed that parathyroidectomy may result in some improvement in CV factors. Nelson et al. found that multiple studies showed improvement in blood pressure, insulin resistance, vascular stiffness, and cardiac morphology following successful surgery [24]. Beysel et al. concluded parathyroidectomy ameliorated the increased CV risk factors in hypercalcemic PHPT [42]. In a study by Bollerslev et al., there is improvement in some aspects of CV dysfunction post-parathyroidectomy [43]. More research is needed on the CV risks with PHPT in post-parathyroidectomy and medical management.
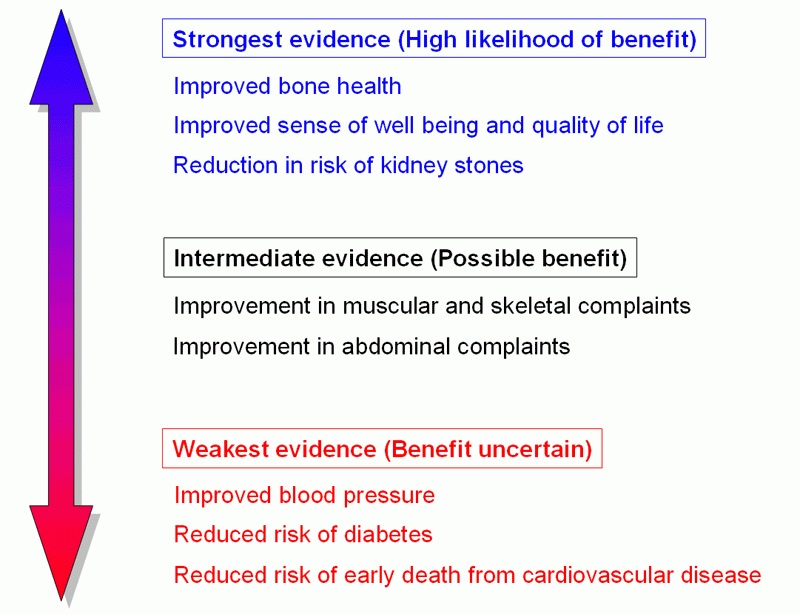
Figure 1: The figure shows the potential benefits of parathyroidectomy to the various symptoms as well as complications of PHPT with the level of evidence for CV complications one of the weakest. UCLA Health https://www.uclahealth.org/endocrine-center/parathyroid-surgery-benefits [44].
The evidence for the medical management of PHPT is consistently inconclusive on the improvement of the CV risk. Medical management of PHPT is usually only for those patients who are asymptomatic and do not meet the criteria for surgery, are unfit, or decline surgery [40]. Untreated asymptomatic PHPT can progress to develop symptoms, and this may further increased CV disease risk [45, 46]. Leere et al. recognized that asymptomatic patients over time might develop complications including CV disease and thus patients who are unable to have surgery should be monitored [47].
NICE advises treatment with cinacalcet for patients with calcium > 2.85 mmol/l with symptoms of hypercalcemia or serum calcium > 3.0 mmo/l with or without symptoms [40]. Leere et al. study showed cinacalcet reduced serum total calcium (87%), and PTH was also seen to decrease [47]. In the systemic review by Leere et al. of forty different studies, he demonstrated that this reduction is only sustained in 8% of the patients [47]. [Bilezikian et al. also found that cinacalcet achieved a long-term reduction in serum calcium and PTH levels [36].
Bisphosphonates are used to reduce fracture risks [40]. Bandeira et al. found the effects on serum were inconsistent [48], Leere et al. noted that it only showed an initial decrease in calcium levels, less than 6 months [47]. Bilezikian et al. study showed treatment with a vitamin D supplement reduced PTH levels and decreased bone turnover with exacerbating hypercalcemia [36].
Based on the various studies, those on medical treatment are more at risk of the progression of their disease and complications of CV disease attributable to rising calcium and PTH levels. If treatment is optimized, then the patient’s condition should improve. It can therefore be concluded that overall, the role of medical therapy in PHPT as far as CV complications are concerned is not evident to be strong, and when compared with surgical treatment, it is far less effective. Also, the cinacalcet effect is stronger than bisphosphonate evidence in terms of the reduction in CV risk. This is based on the efficacy of the medications because there is no study comparing the two with the CV outcomes.
Clinical perspective
The asymptomatic and mild PHPT may be more at risk of CV complications because they are those that still have a dilemma in terms of indications for surgery. These are mostly the normocalcemic patients and they might be protected from the adverse high calcium level but there are mechanisms that showed direct effect of high PTH. Whether these patients should be offered surgery because of these complications is still a matter of debate that is to be answered, as it is obvious that most of these complications are not reversed even after treatment in the future.

