Abstract
Background: Remote monitoring (RM) for cardiac implantable electronic devices (CIED) is a class 1A recommendation by expert consensus. RM incorporation into CIED management allows for early detection of abnormalities, improved patient satisfaction, and enhanced device clinic efficiency.
Cleveland Clinic Abu Dhabi (CCAD) established the first RM program in the United Arab Emirates in 2015, with over 1000 patients enrolled. We report patient RM compliance as an indicator of the overall benefit of our service, aiming to encourage the widespread application of RM technology in the Middle East and other emerging markets.
Objective: To detail the implementation and efficacy of RM service for CIED patients at CCAD to be modeled after as the standard of care for the region.
Methods: Patients are enrolled in RM before hospital discharge and undergo device and wound checks one week later in the device clinic. Additional patient education is provided at that time. Subsequent follow-up includes routine in-person evaluations as well as scheduled RM transmissions. Patient compliance is measured as the proportion of received RM transmissions in relation to all scheduled transmissions.
Results: A total of 1084 patients were enrolled in RM between December 2015 and May 2022. The overall RM compliance rate at 1-year post-implant was 85%.
Conclusion: Our study shows that the establishment of a large-scale RM program in the Middle East region is feasible and associated with excellent patient participation and compliance. We encourage RM integration as the standard of care for CIED patients in our region and areas of the world where such integration has been lagging.
Keywords
remote monitoring, cardiac implantable electronic devices, pacemaker, implantable cardioverter defibrillator, cardiac resynchronization therapy, Middle East
Abbreviations
RM: remote monitoring; CIED: cardiac implantable electronic devices; CCAD: Cleveland Clinic Abu Dhabi; EMR: electronic medical record; ILRs: implantable loop recorders; ICDs: implantable cardioverter defibrillators; PM: pacemaker
Introduction
Remote monitoring (RM) of cardiac implantable electronic devices (CIED) was first introduced in 1971 to complement in-person device assessment [1]. Today, it is widely considered to be the gold standard for care. It has a class 1A indication from the 2021 European Society of Cardiology (ESC) guidelines on cardiac pacing [2, 3].
Over the past decade, many well-designed randomized trials have demonstrated distinct advantages for RM in the management of CIED patients. Clear morbidity and even mortality benefits have been demonstrated across the spectrum of pacemakers, implantable cardioverter defibrillators (ICDs), and biventricular devices. RM allows for the early detection of device abnormalities and actionable patient events. For patients, this has led to fewer hospitalizations, strokes, heart failure exacerbations, and early attention to increasing arrhythmia burdens [4, 5]. Those patient benefits have been realized with the convenience of transmitting from home without exposure to the health risks of physically entering hospital environments (i.e., hospital-acquired infections). The increased ease of use has led to improved levels of patient satisfaction with RM technology [6]. Societies have also benefited from lower hospitalization rates, reduced hospital congestion, and fewer missed workdays by patients [6–9].
RM has also benefited physicians and hospitals in terms of reducing in-person appointments, improving practice efficiency, and reducing clinic congestion [10]. The marked decrease in physical visits (exceeding 50% in some locations) has allowed for more structured workdays [6]. Some regions of the world have had success with clinicians working from home managing RM transmissions, which may help with the recruitment and retention of qualified staff [6]. RM technology has allowed many clinics to outsource some of the RM responsibilities to dedicated commercial entities.
Despite RM’s clear benefits, the establishment of best practices, and recommendations from international guidelines, many parts of the world have not adopted widespread RM use. Some of the frequent challenges and obstacles identified include [6–8]:
- Reluctance of some societies and individuals to accept new technologies.
- Concern by many patients about the loss of face-to-face interaction with their physician/nurse/technologist.
- Concerns by some patients about data safety, including the issue of device hacking and what it means to be “monitored”.
- Legal barriers, including laws governing the privacy of patient medical information.
- Absence of insurance reimbursement schemes for virtual/remote visits.
- Lack of trained hospital personnel to handle increasing volumes of RM data.
- Concerns about the ability to handle potentially overwhelming transition periods from schemes that rely solely on physical visits to those that gradually incorporate virtual RM visits.
- Concerns about delayed attention to critical patient RM transmission data.
- Concerns about handling high volumes of patient telephone calls requesting information about transmissions or reporting technical difficulties.
Unfortunately, these challenges have deterred many institutions from initiating RM programs and adopting the current clear standard of care [7, 8]. In the extended Middle East and Arabian Gulf region, there has been minimal implementation of RM programs.
Despite the many challenges, individual institutions worldwide have demonstrated that such challenges are far from prohibitive. Creating teams with trained personnel, appropriate planning, and organized workflows can lead to the successful implementation of RM in previously unserved areas.
In mid-2015, Cleveland Clinic Abu Dhabi (CCAD) was established as a tertiary referral medical center with a large program focusing on cardiovascular medicine and cardiac care. It immediately adopted RM technology and offered the service to all patients (as a default standard of care). There were initial difficulties that were methodically addressed. By early 2022, the RM program had enrolled over 1000 patients in a well-functioning service. Success required a combination of conviction, flexibility, creativity, and staff dedication.
In this study, we describe how we implemented our RM program and present data on patient acceptance and compliance. The overarching objective is to inspire other institutions (both in our immediate region and around the world) to accept the challenge and implement their own RM programs in order to benefit their patients and societies.
Objective
To detail the implementation of RM service for patients with CIED at CCAD to be modeled after as the standard of care for the Middle East region. Incorporation of RM into CIED management allows for early detection of actionable arrhythmias and device abnormalities. Despite that, RM integration has yet to become the standard of practice in our extended part of the world. As the first and largest RM center in the region, we report on patient RM compliance as an indicator of the overall benefit of our service in order to encourage the widespread application of RM technology in the Middle East and beyond.
Methods
The team
There are four manufacturers of CIED that currently offer RM capability in the United Arab Emirates (UAE); Medtronic, Biotronik, Abbott/St. Jude Medical and Boston Scientific. Prior to 2015, none of the manufacturers had regular RM service in the region. Each manufacturer worked with the local regulatory agency, the Telecommunications and Digital Government Regulatory Authority (TDRA), to obtain the necessary approvals to introduce their RM services. Each manufacturer is responsible for supplying their proprietary monitors to patients, maintaining RM websites, and assisting with patient education and troubleshooting as required.
The RM device clinic is staffed by cardiac electrophysiologists, device technologists who specialize in CIED management, and support staff educated on the basics of RM. Post-implant technologists are responsible for enrolling patients into the RM clinic and ensuring each patient receives appropriate education on how the system/process works. Technologists are also responsible for downloading and assessing all data transmitted from these patient devices. Each transmission is assessed for actionable data and triaged accordingly. Formal reports are generated for all transmissions and entered into our electronic medical record (EMR). Clinic support personnel ensure proper scheduling of both RM transmissions and necessary in-person patient visits. They also provide patients with direct multi-lingual telephonic and electronic messaging access to our clinic. As part of their duties, they send various patient reminders to ensure compliance with RM.
Patient journey
Following the CIED implant, RM is offered to all patients as a standard of care. Each patient is asked to sign a consent form and is subsequently enrolled in RM shortly after implant and usually before discharge from the hospital. Immediate enrolment is targeted to allow the maximum number of patients to start benefitting from RM’s early detection capabilities. Patient and/or their family care providers are instructed on the use of the RM monitor/equipment and on the importance of compliance with the program. Additional patient education resources, such as instructional videos and specialized multi-lingual information pamphlets are provided. Crucially, patients are also provided with direct clinic contact information (dedicated phone number and email address) and encouraged to use such resources.
Post-device implant, patients are scheduled for an in-person device clinic visit within 7–10 days following hospital discharge. In addition to wound and device evaluation, such visits functions to emphasize the importance of RM, answer patient queries about RM-related matters, and troubleshoot any technical issues that may have arisen.
RM transmissions (automated and manual – according to device type) are scheduled monthly for implantable loop recorders (ILRs) and quarterly for other devices. Each patient is provided with a printed schedule of transmissions as a visual reminder. Patient alerts are individually configured on the respective manufacturer’s RM website. Alerts are programmed with a color-coding scheme (green for normal findings, yellow for intermediate severity content, and red for very concerning events/findings). Alerts containing red/yellow flags have a specialized workflow to ensure prioritization of such transmissions that may have actionable events. Such individualization of alert settings is very helpful in prioritizing urgent matters while controlling the amount of incoming device data transmissions and avoiding data overload.
For patients with automated device transmissions, additional manual transmissions can also be arranged on an as-needed basis such as when patients experience certain concerning symptoms. We emphasize to patients, as part of our continuing education process, that RM is not to be used as an emergency system and that transmissions are reviewed during regular office hours. Patients receive written recommendations for how to act in cases of severe symptoms and with certain events such as defibrillator shocks.
Over the next few months, each patient is tracked to assess compliance with RM transmissions. Those who are deemed compliant are subsequently scheduled for routine in-person evaluation visits semi-annually for ICD, and annually for PM and ILR. Such visits are in addition to routine follow-ups with the patient’s primary cardiologist. RM patient compliance is measured as the actual number of transmissions received per year as a proportion of all scheduled transmissions.
Processing of transmissions
Transmissions are reviewed by trained RM technologists. The responsible electrophysiologist is alerted immediately of actionable events. RM technologists allocate early morning dedicated clinic time to review all incoming transmissions while prioritizing those with potential abnormalities. Reports are generated based on specialized templates and are uploaded to our EMR system. Such reports become immediately available to the electrophysiology physician team for easy review and signature.
Reports also become instantly available to other caregivers, including other treating physicians (i.e., heart failure clinic), who may want to adjust certain aspects of treatment based on findings. This aspect of the program was particularly important and useful during the COVID-19 pandemic surges, as our patients were often followed virtually by their primary cardiologists. Those physicians were able to have the latest transmission results with a wealth of information, including data on patient activity level, possible fluid accumulation, and arrhythmia episode counters.
Regular CIED transmissions and report generation also have non-clinical benefits, such as clear and predictable tracking of patient billing and insurance reimbursement. Those financial aspects are crucial to sustaining the functioning and operations of a large device clinic. Integration of RM with EMR allows for timely submission of insurance reimbursement requests (monthly for ILR and quarterly for PPM and ICD).
Analysis
One of the significant challenges we encountered at CCAD was patient acceptance of having RM to complement their in-person device checks. Many patients were accustomed to having regular visits with their physician and felt RM technology would result in a loss of connection to their provider due to the reduced need for clinic visits. Once we established our patient journey, focusing on continued patient education on the importance of RM, we were able to encourage our patients to remain “compliant” with our service, defined as patients transmitting from their remote monitor by a pre-determined number of transmissions annually. Knowing they were better connected to their healthcare providers with the addition of RM increased their willingness to enroll in our program.
In order to evaluate the overall success of our RM program at CCAD, we analyzed patient compliance as an indicator of patient acceptance of our service within the first-year post-device implant. This would demonstrate that patient reluctance to RM technology, as one of the challenges faced, may be overcome with a well-designed RM service and encourage other clinics to initiate their own programs.
Statistics
Microsoft Excel version 2013 was used to analyze the data. Descriptive analysis was used to describe the patient population. Data were reported in terms of mean values with a median. For categorical variables, frequency and percentage were reported.
Results
A total of 1084 patients were enrolled in RM between December 2015 and 5th May 2022. 64% were males, and the average age was 59 years (range 13–100 years). Eighty patients (7.4%) died during follow-up. The majority of devices implanted were PM (366 devices), followed by ILR with 316 implants. There were 218 ICDs and 184 CRTs implanted during this time frame (Figure 1).
Overall RM compliance rate in the 1st year after the implant was 85% (Figure 2). When assessing based on 75% of transmissions received (at least 9 transmissions for ILR and 3 for all other devices), the compliance rate increases to 87%. 90% of the total number of patients were 50% compliant during the 1st year of RM (Figure 3).
Of 154 non-compliant patients, 103 have devices with automated transmissions and 49 needed manual transmission initiated by the patient. Non-compliance rate was higher for devices with manual transmissions (18%) in comparison with the ones with automated transmission (13%) (Figure 4).
When looking at the distribution of compliance rate per device type, we found no significant differences. The non-compliance rate per device type ranges between 12–16% (Figure 4).
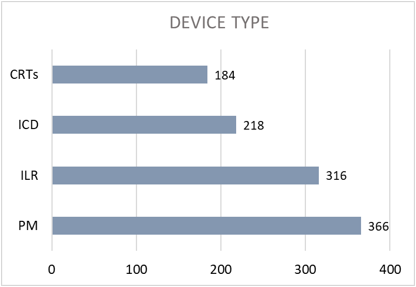 Figure 1: Distribution of the patients as per device type.
Figure 1: Distribution of the patients as per device type.
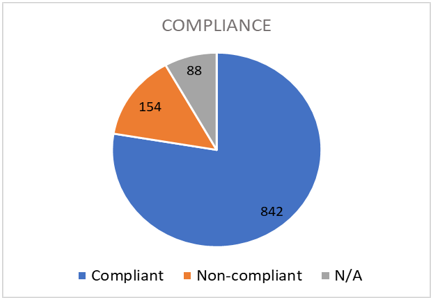 Figure 2: Overall compliance distribution.
Figure 2: Overall compliance distribution.
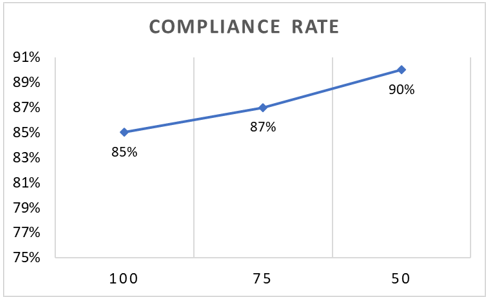 Figure 3: Compliance rate according to the number of transmissions in the 1st year. 100 compliancy means 12 transmissions for ILR and 4 for all other devices, 75 compliancy was ≥9 transmissions for ILR and ≥3 for all other devices, 50 compliancy refers to at least 6 transmissions for ILR and 2 for all other devices. ILR: implantable loop recorder.
Figure 3: Compliance rate according to the number of transmissions in the 1st year. 100 compliancy means 12 transmissions for ILR and 4 for all other devices, 75 compliancy was ≥9 transmissions for ILR and ≥3 for all other devices, 50 compliancy refers to at least 6 transmissions for ILR and 2 for all other devices. ILR: implantable loop recorder.
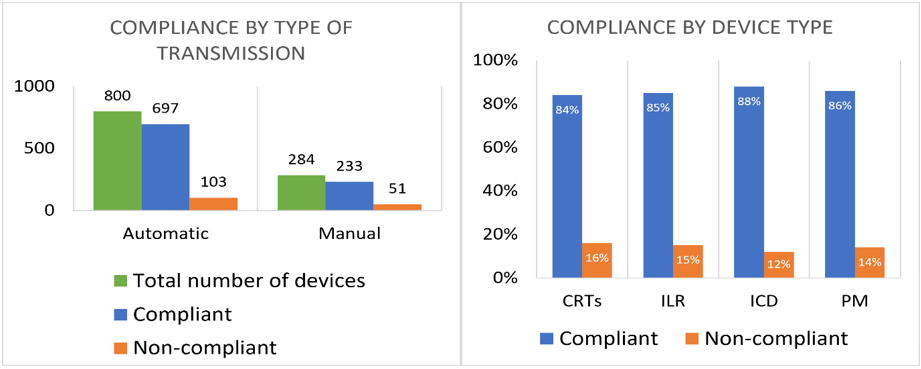 Figure 4: Compliance distribution by type of transmission and by device type.
Figure 4: Compliance distribution by type of transmission and by device type.
Eighty-eight patients (8%) were classified as “N/A” – RM system was not delivered due to several reasons (patient died soon after implant, device explanted due to system infection, heart transplant, etc.). Patients who were implanted less than 1 month for ILR and 3 months for ICD/PPM and did not submit an additional transmission after initial set-up prior to data collection were also classified as “N/A”. These patients were not taken into consideration for the calculation of the compliance rate.
A limitation of our study is that we included patients who had not yet completed a full year of follow-up post-implant, as long as they met the scheduled compliance criteria up until the date of data collection (once every 3 months for ICD/PPM and once monthly for ILR).
Discussion
The initiation of the RM program can be challenging, particularly in a new regulatory and/or geographical environment. However, our results also show with careful planning and patient education, RM programs are successful in achieving excellent patient acceptance and compliance rates.
We elected to initially work with two device manufacturers and eventually expanded to include all vendors willing to offer the service in our region. None of the manufacturers of pacemakers and ICDs had existing systems in place in our region to support RM. They each had to work with our institution and governmental regulatory agencies to build and test the systems needed to support RM. Designing a “patient journey” and related workflows needed to be established before enrolling our first patient. Starting small and building up the service over time allowed us to create a system that has worked well for our clinic and our unique circumstances.
With the initiation of RM services, the cooperation between our hospital and each of the device manufacturers was critical to support long-term staff and patient education activities. It was also essential to support technical troubleshooting issues related to patient enrolment and transmissions. There was a need, at times, to alleviate patient concerns about data privacy and data security (i.e., hacking concerns).
Device company representatives also helped optimize internal clinic workflows, such as programming clinic software and interfaces to categorize and flag the different types of alerts. The extensive interactions, especially during the first year of operations, helped tame many of the technological barriers experienced by clinicians as they were enrolling patients and assessing their transmissions.
We did not use or consult with any third-party providers (such as companies to which RM operations can be outsourced to a great extent). However, other institutions considering initiation of RM operations with limited staffing resources may benefit from doing so.
Enrolling patients soon after the implant is important for the early detection of abnormalities and arrhythmias. Investing significant time and effort into patient education at the time of enrolment (with clear instructions on RM use, benefits, monitoring hours, and clinic contact information) helped alleviate many patient concerns regarding the use of new technology, loss of face-to-face interaction, and data privacy. Continuous patient education was supported with direct clinic contact information, printed materials, and short educational videos (in multiple languages), which was essential in our achievement of excellent patient acceptance and compliance rates.
Systems were also put in place to quickly identify patients who were non-compliant with RM. Each RM vendor site displays lists of “missed transmissions” and “disconnected monitors”. Reminder phone calls, messages, and add-on in-person visits were utilized to improve compliance and enhance patient acceptance of the technology.
Long-term efforts to reiterate patient education, and to schedule in-person “as-needed” device clinic visits, have resulted in an overall compliance rate at our institute of 85%.
It is very important for patients to understand that RM services do not constitute continuous “live” monitoring of patients. Some patients and family members have continued to believe that hospital staff were monitoring patients in a manner similar to live telemetry. Efforts to explain how RM works are essential, in order to properly set patient expectations and to prevent misunderstandings. Similarly, patients need to be clear that manual transmissions (especially when sent on an urgent basis) may not be viewed by clinical staff immediately (depending on the time of transmission and even the day of the week). As technology continues to advance and as EMR integration becomes more feasible, patients may be able to receive feedback on whether their transmissions were received and if any action is needed.
It is also important to explain to patients that RM services complement their in-person visits but do not completely replace them. There is still a role for in-person visits in the device clinic, as well as with the treating physician, to address clinical patient issues. Taking the time, often repeatedly, to explain the role of RM in the overall patient treatment plan is worth the time investment.
Transmission reporting via RM can reduce the amount of time spent by clinic staff per patient when compared to the overall time commitment when patients are seen in person. As a patient repeatedly has relatively normal transmissions, the number of in-person visits can be kept to a minimum. This is a great benefit for patients who live at a great distance from specialized hospitals, as well as for elderly patients who face difficulty with ambulating outside their homes. During the initial COVID-19 pandemic waves, RM played a very important role in allowing patients to be monitored from the safety of their homes.
Reimbursement for RM services is another challenge that needs to be overcome. We were able to work with the local Department of Health to adopt/activate previously unused billing codes. Working with insurance companies, often to educate their personnel on what RM is and how it works, led to a significant reduction in insurance claim denials over time. Part of the education is centered around explaining the benefit of RM, including cost reductions from early detection of health problems (i.e., heart failure exacerbations), that RM services provide.
Finally, operating a well-run RM system has allowed us to maintain a database of patient and device information. Such databases allow us to keep better track of our patients and to respond with more ease and agility in scenarios involving device recalls and alerts.
Conclusion
We demonstrate the efficacy of the implementation of RM service for patients as a viable and worthy strategy. To ensure the effectiveness of such a service, it is necessary to invest in a skilled and dedicated multidisciplinary team and to provide them with clear monitoring and action protocols. Using our model as a guide and our high patient compliance with our service, we hope to encourage integrating RM as the standard of care for patients with CIED in the Middle East region and beyond.
Limitations
A limitation of our study is that our center has a well-established team dedicated to RM, and therefore other programs with fewer resources may not initially experience similar results.
Author Contributions
Almuti K: conceptualization, methodology, writing – reviewing and editing; Pais R: investigation, data curation; da Costa S: writing – original draft preparation; Gancinho A: original draft preparation; McCutchon K: supervision, investigation, writing – original draft, writing – reviewing and editing; AlJaabari M: writing – reviewing and editing; Shafiei F: writing – reviewing and editing.
Patient Consent
As a retrospective data analysis for remote monitoring compliance, the need for patient consent was waived.
Ethics Statement
The methods comply with the principles of the Helsinki Declaration. Ethics approval was granted by the REC committee at CCAD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure/Conflicts of Interest
Almuti K, AlJaabari M, Shafiei F have nothing to disclose; McCutchon K, consultant, Medtronic; da Costa S, Pais R, and Gancinho A have nothing to disclose. The authors report no relationships that could be construed as a conflict of interest.
References
- Slotwiner D, Varma N, Akar JG, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12(7):e69-100.
- Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427-520.
- Chugh SS. Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy, 5th Edition. By Kenneth A. Ellenbogen, Bruce L. Wilkoff, G. Neal Kay, Chu-Pak Lau, and Angelo Auricchio. Philadelphia, PA: Elsevier, Inc., 1232 Pages, ISBN: 978-0-323-37804-8. BOOK REVIEW. Pacing Clin Electrophysiol. 2017;40(2):221.
- Zeitler EP, Piccini JP. Remote monitoring of cardiac implantable electronic devices (CIED). Trends Cardiovasc Med. 2016;26(6):568-77.
- Lim PC, Lee AS, Chua KC, et al. Remote monitoring of patients with cardiac implantable electronic devices: a Southeast Asian, single-centre pilot study. Singapore Med J. 2016;57(7):372-77.
- Ricci RP, Morichelli L, Varma N. Remote Monitoring for Follow-up of Patients with Cardiac Implantable Electronic Devices. Arrhythm Electrophysiol Rev. 2014;3(2):123-28.
- Maines M, Tomasi G, Moggio P, et al. Implementation of remote follow-up of cardiac implantable electronic devices in clinical practice: organizational implications and resource consumption. J Cardiovasc Med. 2020;21(9):648-53.
- Mairesse GH, Braunschweig F, Klersy K, et al. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics committee of the European Heart Rhythm Association. Europace. 2015;17(5):814-18.
- Tarakji KG, Zaidi AM, Zweibel SL, et al. Performance of first pacemaker to use smart device app for remote monitoring. Heart Rhythm O2. 2021;2(5):463-71.
- Gustafsson PE. Implementing remote monitoring of cardiac implantable electronic devices: the clinical experience from one center in Sweden. The Journal of Innovations in Cardiac Rhythm Management. 2014;5:1733-39.
![]() *, da Costa S, Pais R, Gancinho A and Shafiei F
*, da Costa S, Pais R, Gancinho A and Shafiei F



