Withers K*1, Raby K2, Parker W 1, German C
1, German C 3, Eyadiel L
3, Eyadiel L 4, Hilton T
4, Hilton T 5, Goslen K1, Green S6, Banoian D7, Bapat S1, Thomas A1, Seals A
5, Goslen K1, Green S6, Banoian D7, Bapat S1, Thomas A1, Seals A 4 and Pisani B4
4 and Pisani B4
1Department of Internal Medicine, Atrium Health Wake Forest Baptist, Winston-Salem, NC, USA
2Section of Cardiology, Medical University of South Carolina, Charleston, SC, USA
3Section of Cardiology, The University of Chicago Pritzker School of Medicine, Chicago, IL, USA
4Section of Cardiology, Atrium Health Wake Forest Baptist, Winston-Salem, NC, USA
5Department of Internal Medicine, University of Virginia, Charlottesville, VA, USA
6Department of Anesthesiology, Cleveland Clinic Florida Hospital, Weston, FL, USA
7Department of Internal Medicine, Loma Linda University Medical Center, Loma Linda, CA, USA
*Correspondence: Kaitlyn Withers, Department of Internal Medicine, Atrium Health Wake Forest Baptist, Winston-Salem, NC, USA
Received on 08 January 2024; Accepted on 20 February 2024; Published on 27 February 2024
Copyright © 2024 Withers K, et al. This is an open-access article and is distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction: Cardiogenic shock (CS) and acute decompensated heart failure (ADHF) are states of low cardiac output that manifest as tissue hypoperfusion and end-organ damage. Current guidelines do not provide recommendations regarding beta blocker (BB) use in CS. This study sought to analyze the effects of BB continuation on in-hospital mortality among patients admitted with ADHF necessitating inotropic therapy.
Methods: A single-center, retrospective study was conducted in adult patients hospitalized with ADHF requiring inotropes over a three-year period. Patients with CS were stratified based on the Society for Cardiovascular Angiography and Interventions (SCAI) classification of CS and Get With The Guidelines-Heart Failure (GWTG-HF) risk score. BB continuation was assessed at admission and was defined as administration for at least 50% of the hospital stay. A time-to-event analysis framework was implemented using Cox proportional hazards models to analyze the effect of BB continuation on in-hospital mortality.
Results: A total of 449 patients were included in the study. Twelve patients were excluded, as there was insufficient data to calculate the GWTG-HF risk score. A significant mortality benefit was seen with BB continuation in all statistical models. When adjusted for SCAI and GWTG-HF risk score, BB continuation was associated with a 65% reduction in in-hospital mortality [HR 0.35 95% CI 0.19-0.64, p = 0.0008].
Conclusion: In select patients with ADHF resulting in CS, there appears to be a mortality benefit associated with continuation of BB on admission that remains significant after adjustment for severity of CS.
Keywords
cardiogenic shock, beta blocker, acute decompensated heart failure
Abbreviations
HF: heart failure; CS: cardiogenic shock; ADHF: acute decompensated heart failure; BB: beta blocker; SCAI: Society for Cardiovascular Angiography and Interventions; GWTG-HF: Get With The Guidelines-Heart Failure; EMR: electronic medical record; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; VAD: ventricular assist device; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; eGFR: estimated glomerular filtration rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LOS: length of stay; SD: standard deviation
Introduction
Heart failure (HF) is one of the leading causes of morbidity and mortality, with recent estimates showing that over 37 million people are affected globally, five million of whom are in the United States [1]. Despite medical advances, acute decompensated heart failure (ADHF) is an increasingly common reason for hospitalization, especially in people over the age of 65 [2]. In its most severe form, ADHF can present as cardiogenic shock (CS), a state of low cardiac output that manifests as tissue hypoperfusion, which frequently requires inotropic support [3]. These disease states have a substantial public health impact, with estimated post-discharge one-year mortality as high as 30% and 50% for ADHF and CS, respectively [4, 5].
There is a robust pool of data demonstrating the survival benefit of beta blocker (BB) therapy in chronic HF [6–8]. Despite recommendations for use in chronic HF by national and international guidelines [9], only 67% of patients with HF and a reduced ejection fraction are on BB therapy [10]. Hospitalizations for HF present an opportunity for clinicians to initiate and optimize guideline-directed medical therapy [3]. However, due to concern that BB are negative inotropes that may worsen outcomes in ADHF, these medications are often dose-reduced or discontinued in the acute setting [11]. This action is not supported by current literature, which suggests up to a 3.28-fold increase in in-hospital mortality for patients with ADHF whose BB were discontinued upon hospital admission [11–13].
The effect of BB therapy in CS is less clear, and clinicians commonly face the dilemma of stopping BB when initiating inotropes. Historically, CS diagnostic criteria lacked uniformity, limiting the evaluation and treatment of an already heterogeneous patient population [5]. The advent of validated qualitative and quantitative measures of disease severity, such as the Society for Cardiovascular Angiography and Interventions (SCAI) classification of CS and Get With The Guidelines-Heart Failure (GWTG-HF) risk score, may help to standardize practice patterns, severity, and staging of CS [14–16]. Given the previously demonstrated benefits in chronic and ADHF [6–8, 12, 13], we hypothesized that BB continuation may provide an advantage in CS patients requiring hospitalization. To test this hypothesis, we performed a retrospective analysis of outcomes among patients admitted with ADHF requiring inotrope support.
Methods
This was a single-center, retrospective study performed at Atrium Health Wake Forest Baptist Medical Center, a tertiary health care facility based in Winston-Salem, North Carolina. Data from the electronic medical record (EMR) was obtained to identify patients with ADHF [Heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF)] requiring inotropes over a three-year period, from 1/1/2017–1/1/2020 using ICD codes I50.23, I50.43, I50.9, R57.0, I50.20, I50.21, I50.41, I50.82, and I50.814. Inclusion criteria were patients ≥18 of age admitted with ADHF who required inotropic therapy with either milrinone or dobutamine. Exclusion criteria were pre-and post-operative patients, patients with temporary and durable mechanical circulatory support [ventricular assist device (VAD)], and post-heart transplant recipients. The study received approval from the Institutional Review Board of Atrium Health Wake Forest Baptist Medical Center.
The predictor variable for this analysis was BB status on admission. Continuation of BB was defined as BB administered for at least 50% of the patient’s hospital stay at the same home dose or a reduced dose. BB administration that did not meet this definition was considered BB discontinuation.
The baseline data set included age, sex, race/ethnicity, history of hypertension (on antihypertensive treatment and/or systolic blood pressure >130 mm Hg), history of type I or type II diabetes (on therapy and/or hemoglobin A1c > 6.5%), smoking status, body mass index (BMI) (weight in kilograms divided by height in meters squared), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and left ventricular ejection fraction (LVEF). Patient’s systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), estimated glomerular filtration rate (eGFR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate were reported values obtained prior to initiation of inotrope therapy. Inotrope therapy included either milrinone or dobutamine. The choice of inotrope agent and dose was based on provider preference and clinical factors relevant to patient care. Additionally, SCAI stage and GWTG-HF scores were calculated at the time of admission.
The primary outcome considered in the analysis was in-hospital mortality. In-hospital mortality was assessed using the EMR and a review of the discharge or death note for each patient. Length of stay (LOS) was obtained from the EMR and defined as the number of days between the admission and discharge date.
The total cohort was dichotomized based on BB status on admission. The baseline demographic characteristics, LOS, and mortality were assessed for each group. Continuous variables were documented as a mean and standard deviation (SD). Categorical variables were documented as a total number (n) and percentage (%). Chi-square test and t-tests were used to test for differences in demographic/covariate distributions between the two groups of interest.
Cox proportional hazards models were used to assess the association between the continuation of BB on admission and in-hospital mortality. In addition, simple and multiple linear regression was used to assess the association between the continuation of BB and LOS. Model 1 represents the unadjusted relationship. Model 2 adjusted for age, race/ethnicity, and sex. Model 3 additionally adjusted for history of hypertension, history of diabetes, smoking status, BMI, SBP, DBP, HR, HDL-C, TC, eGFR, AST, ALT, lactate, inotrope type (milrinone or dobutamine), and inotrope duration. Model 4 adjusted for SCAI stages and GWTG-HF scores. There was an insufficient sample size to create a Cox proportional hazard model stratified by inotrope type. Variables that contained missing values were GFR, AST, ALT, and lactate. To account for this during multivariate modeling, multiple imputation was implemented, with effects pooled across 100 fully imputed datasets. This was done using packages “mice” and “Hmisc” in R. Survival curves were created to visualize differences in in-hospital mortality between groups and analyzed using the Wilcoxon Group Homogeneity Test. All analyses were completed using R (ver 4.0.2). A p-value of < 0.05 was considered statistically significant.
Results
A total of 449 patients were admitted with ADHF requiring inotropes who met the study criteria. Of these, 206 (45.9%) were continued BB therapy. Demographic data, clinical history, and clinical data at the time of inotrope therapy are reported in the table below (Table 1). 69% of patients were male, and 64.1% were non-Hispanic white. Patients whose BB was continued were more likely to be younger, male, have a history of hypertension, and have higher SBP, DBP, HR, TC, HDL, AST, and ALT. A total of 263 patients were initiated on milrinone, 128 patients were on dobutamine, and 58 were on both agents. Of those, 57.8%, 32.8%, and 20.7% were continued on BB therapy. There was a total of 81 (18%) patient deaths. Of those, 68 had their BB discontinued on admission, while the remaining 13 were continued on BB therapy. The mortality rate was higher in those who discontinued BB therapy compared with those in the BB continuation group at 28% and 6.31%, respectively (p < 0.0001). The figure demonstrates the in-hospital mortality benefit of continuation of BB therapy (Figure 1).
| Characteristics | Beta blocker status | Missing n (%) | p-value |
| | Continued N = 206 | Discontinued N = 243 | | |
| Age, mean (±SD) | 61.2 (13.1) | 65 (14.6) | 0 | 0.0045 |
| Male, n (%) | 150 (72.8) | 158 (65) | 0 | 0.0471 |
| Non-Hispanic white, n (%) | 121 (58.7) | 167 (68.7) | 0 | 0.0872 |
| Hypertension, n (%) | 177 (85.9) | 191 (78.6) | 0 | 0.0444 |
| Diabetes mellitus, n (%) | 75 (36.4) | 84 (34.6) | 0 | 0.6846 |
| Current smoker, n (%) | 16 (7.8) | 34 (14.1) | 0 | 0.1871 |
| BMI, kg/m2, mean (± SD) | 31.12 (7.54) | 29.93 (8.7) | 0 | 0.1189 |
| Systolic BP, mm Hg, mean (± SD) | 110.4 (20.3) | 103.5 (20.6) | 0 | < 0.001 |
| Diastolic BP, mm Hg, mean (± SD) | 71.6 (16.4) | 67.8 (17.4) | 0 | 0.019 |
| Heart rate, bmp, mean (± SD) | 84.1 (17.7) | 91.1 (18.7) | 0 | < 0.001 |
| HDL, mg/dL, mean (± SD) | 61.49 (51.9) | 54.01 (37.5) | 0 | 0.0429 |
| Total cholesterol, mg/dL, mean (± SD) | 248.03 (194.5) | 207.38 (139.4) | 0 | 0.0127 |
| eGFR, mL/min, mean (± SD) | 45.4 (18.2) | 39.0 (19.2) | 2 (0.4) | < 0.001 |
| AST, U/L, mean (± SD) | 78.6 (213.2) | 466.5 (1614.8) | 24 (5.3) | 0.001 |
| ALT, U/L, mean (± SD) | 78.2 (199.3) | 245.3 (736.3) | 24 (5.3) | 0.002 |
| Lactate, mmol/L, mean (± SD) | 2.8 (2.8) | 3.4 (3.2) | 176 (39.2) | 0.108 |
| Milrinone only, n (%) | 152 (73.7) | 111 (45.7) | 0 | < 0.001 |
| Dobutamine only, n (%) | 42 (20.4) | 86 (35.4) | 0 | – |
| Both inotropes, n (%) | 12 (5.8) | 46 (18.9) | 0 | – |
Table 1: Baseline characteristics. SD = standard deviation; BMI: body mass index; BP: blood pressure; HDL: high-density lipoprotein; eGRF: estimated glomerular filtration rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
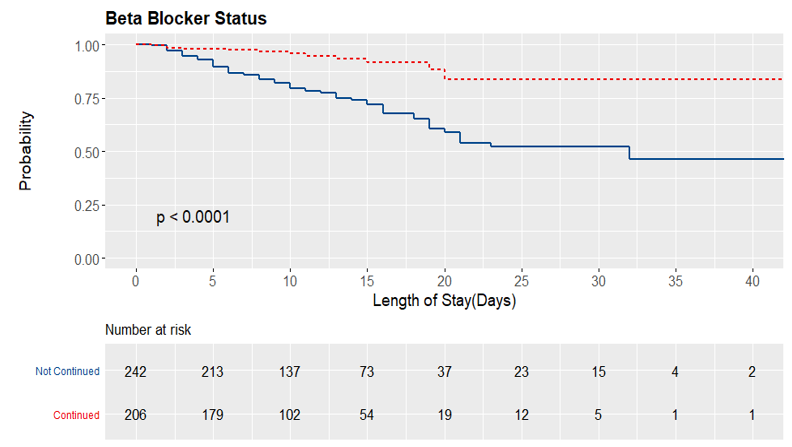
Figure 1: Survival curve demonstrating in-hospital mortality benefit with continuation of BB therapy.
The table displays the distribution of SCAI and GWTG-HF scores amongst the cohort (Table 2). There was insufficient data to calculate GWTG-HF scores in 12 patients, and they were excluded from model 4. The mean GWTG-HF score was 44.29, and 52% of the patients were SCAI stage C or higher upon presentation. The mean GWTG-HF score was significantly higher in the BB discontinued group compared to the BB continued group at 47.12 and 40.98 (p-value < 0.0001). BB status was similar for patients in SCAI stage C. Patients were more likely to have BB continued in SCAI stages A or B and discontinued in stages D or E. Higher SCAI and GWTG-HF scores were associated with an increased risk of in-hospital mortality (Table 2).
| Beta blocker status | |
| Continued | Discontinued | HR (95% CI) |
| GWTG-HF risk score |
| Mean score (± SD) | 40.98 (8.30) | 47.12 (8.54) | – |
| 0-33 [n (%)] | 40 (19.42%) | 13 (5.35%) | Reference level |
| 35-50 [n (%)] | 135 (65.53%) | 145 (59.67%) | 2.56 (0.61-10.71, p = 0.1967) |
| 51-57 [n (%)] | 21 (10.19%) | 49 (20.16%) | 3.78 (0.86-16.53, p = 0.0773) |
| >/=58 [n (%)] | 5 (2.43%) | 49 (11.93%) | 5.36 (1.17-24.55, p = 0.0306) |
| SCAI stages |
| A or B [n (%)] | 107 (51.94%) | 106 (43.62%) | Reference level |
| C [n (%)] | 90 (43.69%) | 104 (42.80%) | 1.49 (0.90-2.47, p = 0.1219) |
| D or E [n (%)] | 9 (4.37%) | 33 (13.58%) | 2.66 (1.46-4.85, p = 0.0013) |
Table 2: Risk stratification scores. SD: standard deviation; HR: hazard ratio; CI: confidence interval; GTWG-HF: Get With The Guidelines-Heart Failure; SCAI: Society for Cardiovascular Angiography and Interventions.
Across all four Cox regression models, BB continuation was associated with a decreased risk of in-hospital mortality (Table 3). After adjusting for co-morbid conditions and baseline laboratory values, a 72% reduction in in-hospital mortality was observed [HR 0.28 95% CI 0.14-0.52 p = 0.0001]. This benefit was attenuated but remains significant after adjusting for both the SCAI stage and GWTG-HF score [HR 0.35 95% CI 0.19-0.64 p = 0.0008].
| HR (95% CI) | p-value | LOS difference (days) |
| Model 1 | 0.25 (0.14-0.46) | < 0.0001 | -1.3 (p = 0.0785) |
| Model 2 | 0.27 (0.15-0.49) | < 0.0001 | -1.5 (p = 0.0511) |
| Model 3 | 0.28 (0.14-0.52) | 0.0001 | -1.2 (p = 0.0932) |
| Model 4 | 0.35 (0.19-0.64) | 0.0008 | – |
Table 3: Cox regression models demonstrating decreased risk of in-hospital mortality with BB continuation. Additionally, linear regression models demonstrate a shorter LOS with BB continuation. LOS = length of stay; HR: hazard ratio; CI: confidence interval. Model 1 unadjusted. Model 2 adjusted for age, sex, and race/ethnicity. Model 3 adjusted for model 2 plus hypertension, diabetes, smoking status, BMI, HR, SBP, DBP, HDL-C, TC, eGFR, AST, ALT, lactate, inotrope type, and duration of inotropes. Model 4 adjusted for SCAI and GWTG-HF risk score.
Without adjustment, there was a trend towards longer LOS in the BB discontinuation cohort vs. patients who had BB continued, averaging 1.3 days longer (p = 0.0785). After adjustment for age, race/ethnicity, and sex (Model 2) and adjustment in co-morbid conditions (Model 3), there continued to be a trend toward a shorter LOS in patients who were continued on BB therapy (1.5 days and 1.2 days, respectively), but was not statistically significant (Table 3).
The table demonstrates the outcomes of patients stratified by inotrope type (milrinone vs. dobutamine) (Table 4). A total of 65 patients (14.5%) experienced in-hospital mortality. There was a statistically significant difference in in-hospital mortality in those who received dobutamine compared to milrinone [28.9% and 10.6%, respectively (p < 0.001)]. There was no difference in LOS between the two groups.
| Dobutamine N = 128 | Milrinone N = 263 | p-value |
| In-hospital mortality, n (%) | 37 (28.9) | 28 (10.6) | < 0.001 |
| LOS, mean (± SD) | 11.2 (8.1) | 11.1 (6.6) | 0.923 |
Table 4: Outcomes by inotrope therapy. SD: standard deviation; LOS: length of stay.
Discussion
This study compared the outcomes in patients continued on BB therapy with patients whose BB therapy was discontinued during hospitalization for ADHF requiring inotrope therapy. There were two main findings. First, patients had a lower risk of in-hospital mortality with the continuation of BB therapy. Second, hospital LOS trended towards a shorter stay when BB was continued at the time of admission.
While BB have proven to be safe and effective for patients with chronic HF, the clinical effect of using BBs and inotropic therapy together in the acute setting is largely unknown [6–8]. Inotropes such as dobutamine and milrinone are commonly employed to improve cardiac output in patients with SCAI stage C CS. However, this comes at the cost of increasing the risk of tachyarrhythmias and increasing myocardial oxygen consumption, potentially increasing mortality in this population [17]. Early hemodynamic studies demonstrated a blunted response to inotropes when paired with BB, leading to guidelines that caution against using BB in combination with inotropes [3, 18]. However, there is an increasing body of evidence that suggests BB therapy may be safe when used concurrently with inotropic medication. Furthermore, there may be an increased mortality with BB withdrawal in the setting of milrinone administration [19]. BB have demonstrated favorable effects when paired with inotropes, leading to HR reduction and a trend towards fewer arrhythmias without impacting cardiac output [20, 21]. Additionally, patients continued on BB therapy are more likely to remain on BB, and at higher doses, upon hospital discharge [22, 23]. These studies are limited by their retrospective nature and small sample size, but they suggest BB are safe to continue in selected patients with CS requiring inotropes.
Milrinone is also utilized in the outpatient setting as a palliative tool or as a bridge to advanced HF therapies, such as VAD or heart transplants. When clinically stable, patients with severe HF have demonstrated the ability to tolerate BB therapy while on continuous milrinone [24, 25]. One study assessed outcomes in patients on ambulatory inotrope therapy with and without concomitant use of BBs. They found a significant reduction in HF hospitalizations and ventricular arrhythmia in the BB group, leading to a longer overall duration of therapy with chronic inotropes [26]. While this adds to the body of literature that BB are safe when used concurrently with inotropes, it has limited generalizability to the acutely decompensated hospitalized patient, given hemodynamic differences. Further, the current literature does not address overall mortality or concomitant use of dobutamine with BB [26].
It is possible that BB discontinuation and the subsequent increased risk of in-hospital mortality are confounded by the severity of the underlying HF. To control for this variable, we controlled for the SCAI stage and GWTG-HF risk score at the time of admission. The SCAI classification system for CS has been validated across multiple CS phenotypes and correlates with short and long-term mortality [16]. Patients are graded by level of congestion, end-organ perfusion, laboratory abnormalities, hemodynamic alterations, and the need for vasopressors or inotropes [16]. Similarly, the GWTG-HF risk score relies on co-morbid conditions, age, vital signs, and labs – that are readily available at the time of admission, with a higher score indicating worse severity of illness and has been validated to correlate with mortality [15]. Supporting prior studies, we found a significant association between in-hospital mortality and higher CS stage or risk score on hospital presentation. Our patient population represents a critically ill cohort, with over half defined as SCAI stage C or higher on initial presentation. Clinicians were more likely to stop BB therapy based on the severity of illness, as evidenced by higher GTWG-HF and SCAI scores, abnormal liver function tests, and lower blood pressures, as it seems intuitive that patients in a low-output state and hypotension would not tolerate these medications. Importantly, BB discontinuation was determinantal, and despite adjustment for CS severity, patients had a significantly higher risk for in-hospital mortality if BB therapy was discontinued at admission. Furthermore, the patients whose BB were discontinued were also found to have a trend towards longer hospital LOS, which resulted in higher hospitalization costs.
Lastly, this study demonstrated that there was significantly higher in-hospital mortality within the dobutamine group regardless of BB therapy. BB discontinuation was more common in patients started on dobutamine, although 20.4% of patients remained on BB in this group, compared to patients initiated on milrinone (Table 1). While this study was unable to assess mortality based on specific inotrope when stratified by BB continuation, it does raise the concern of whether BB therapy contributed to the observed outcome.
Overall, our study suggests that chronic BB therapy may be continued in selected patients admitted with ADHF requiring inotropic therapy. As BB alters the neurohormonal pathway, the mechanism of increased mortality is possibly related to beta-receptor hypersensitivity upon BB withdrawal [27]. Due to a resultant sympathetic surge, the failing heart may be adversely affected by increased myocardial oxygen demands and the risk of ventricular arrhythmia [27]. This is hypothesis-generating, however, and further randomized clinical trials are needed to identify the mechanism of BB’s potential protective effects in CS.
Strengths of this study include a diverse population with representation from both non-whites and females, patients with both HFrEF or HFpEF and patients receiving milrinone or dobutamine. Limitations include the retrospective, single-center design of the study. This study primarily focused on patients who clinically required inotropic therapy as an indicator of CS, as commonly occurs on hospital admission, rather than delaying initiation of inotropes in order to define CS shock based on hemodynamics. Due to the sample size, there was a lack of power to compare outcomes of BB continuation in patients on dobutamine vs. milrinone. Confounding factors were controlled, but residual confounders may have affected our results and conclusions. Although patients with HFrEF and HFpEF were included, there were inadequate numbers of HFpEF patients to examine differences by HF phenotype.
Conclusion
Among patients admitted to the hospital with ADHF requiring inotropic therapy, BB continuation was associated with a reduction in in-hospital mortality and a trend toward shorter LOS. Randomized clinical trials of patients with CS are needed to confirm this association and determine the optimal inotrope and appropriate dose.
Acknowledgments
There were no further contributions to this project beyond those of the listed authors.
Conflicts of Interest
The authors whose names are listed certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368-78.
- Abdo AS. Hospital Management of Acute Decompensated Heart Failure. Am J Med Sci. 2017;353(3):265-74.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032.
- Kurmani S, Squire I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr Heart Fail Rep. 2017;14(5):385-92.
- Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019;8(8):e011991.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-007.
- Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-199.
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200.
- Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-66.
- Abi Khalil C, Al Suwaidi J, Singh R, et al. Beta-Blockers are Associated with Decreased In-Hospital Mortality and Stroke in Acute Decompensated Heart Failure: Findings from a Retrospective Analysis of a 22-Year Registry in the Middle East (1991-2013). Curr Vasc Pharmacol. 2017;15(1):77-83.
- Orso F, Baldasseroni S, Fabbri G, et al. Role of beta-blockers in patients admitted for worsening heart failure in a real world setting: data from the Italian Survey on Acute Heart Failure. Eur J Heart Fail. 2009;11(1):77-84.
- Tamaki Y, Yaku H, Morimoto T, et al. Lower In-Hospital Mortality With Beta-Blocker Use at Admission in Patients With Acute Decompensated Heart Failure. J Am Heart Assoc. 2021;10(13):e020012.
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29-37.
- Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25-32.
- Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022;79(9):933-46.
- Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014;63(20):2069-078.
- Metra M, Nodari S, D’Aloia A, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40(7):1248-258.
- Gattis WA, O’Connor CM, Leimberger JD, et al. Clinical outcomes in patients on beta-blocker therapy admitted with worsening chronic heart failure. Am J Cardiol. 2003;91(2):169-74.
- Di Santo P, Mathew R, Jung RG, et al. Impact of baseline beta-blocker use on inotrope response and clinical outcomes in cardiogenic shock: a subgroup analysis of the DOREMI trial. Crit Care. 2021;25(1):289.
- Kobayashi S, Susa T, Tanaka T, et al. Low-dose β-blocker in combination with milrinone safely improves cardiac function and eliminates pulsus alternans in patients with acute decompensated heart failure. Circ J. 2012;76(7):1646-653.
- Jondeau G, Neuder Y, Eicher JC, et al. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J. 2009;30(18):2186-192.
- Lima MV, Cardoso JN, Ochiai ME, et al. É necessário suspender o betabloqueador na insuficiência cardíaca descompensada com baixo débito? [Is it necessary to suspend betablockers in decompensated heart failure with low output?]. Arq Bras Cardiol. 2010;95(4):530-35.
- Shakar SF, Abraham WT, Gilbert EM, et al. Combined oral positive inotropic and beta-blocker therapy for treatment of refractory class IV heart failure. J Am Coll Cardiol. 1998;31(6):1336-340.
- Earl GL, Verbos-Kazanas MA, Fitzpatrick JM, et al. Tolerability of beta-blockers in outpatients with refractory heart failure who were receiving continuous milrinone. Pharmacotherapy. 2007;27(5):697-706.
- Zaghlol R, Ghazzal A, Radwan S, et al. Beta-blockers and Ambulatory Inotropic Therapy. J Card Fail. 2022;28(8):1309-317.
- Gilligan DM, Chan WL, Stewart R, et al. Adrenergic hypersensitivity after beta-blocker withdrawal in hypertrophic cardiomyopathy. Am J Cardiol. 1991;68(8):766-72.
![]() 1, German C
1, German C![]() 3, Eyadiel L
3, Eyadiel L![]() 4, Hilton T
4, Hilton T![]() 5, Goslen K1, Green S6, Banoian D7, Bapat S1, Thomas A1, Seals A
5, Goslen K1, Green S6, Banoian D7, Bapat S1, Thomas A1, Seals A![]() 4 and Pisani B4
4 and Pisani B4
