Bolognesi M *
*
Head of Centre for Sports Cardiology, AUSL Della Romagna, Cesena, Italy
*Correspondence: Massimo Bolognesi, Head of Centre for Sports Cardiology, AUSL Della Romagna, Cesena, Italy
Received on 22 February 2023; Accepted on 23 March 2023; Published on 03 April 2023
Copyright © 2023 Bolognesi M. This is an open-access article and is distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The safety of exercise in athletes with hypertrophic cardiomyopathy (HCM) and the emphasis on shared decision-making has led to the updating of the most recent recommendations, which have reduced restrictions on exercise and competitive sports activity in this population. This case report confirms that obstructive HCM reduces fitness in the amateur athlete, even if paucisymptomatic or asymptomatic, leading to a physical limitation to practicing high-intensity sports. It also points out how it is possible to differentiate physiological left ventricular (LV) hypertrophy from morphologically mild but pathological one, outlining the clinical aspects from a morphological and functional point of view.
Keywords
hypertrophic cardiomyopathy, athletes, sudden cardiac death, ECG
Abbreviations
HCM: hypertrophic cardiomyopathy; LV: left ventricular; IVS: interventricular septum; CMR: cardiac magnetic resonance; SCD: sudden cardiac death
Introduction
One of the most common heterogeneous hereditary heart diseases is hypertrophic cardiomyopathy (HCM), which consists of various subtypes but is predominantly characterized by an asymmetric left ventricular (LV) wall thickness predominantly of the interventricular septum (IVS) ≥15 mm in the absence of aortic valvular pathology or arterial hypertension, disproportionate to loading conditions [1, 2].
The combined prevalence of clinically expressed HCM and gene carriers was recently estimated at 1:200 [3], although it is likely to be lower in highly trained athletes, as the structural and functional alterations associated with this disorder select many individuals from competitive sports activity [4]. Sports activity has been known to be associated with the risk of sudden cardiac arrest (SCA) in individuals with underlying cardiovascular disease [5], and HCM has been found to be the most common cause of death in young athletes [6, 7]. Consequently, all recommendations from scientific societies have always prevented individuals with clinically evident HCM from taking part in most competitive sports [8]. Although this paradigm has evolved, diagnosis of HCM in apparently healthy paucisymptomatic athletes with a baseline ECG in the normal range is still a challenge for sports medicine. The literature reports that about 1 in 500 people suffer from this pathological situation. However, a recent report taking into account genetic background and new information from cardiac magnetic resonance (CMR) gave a figure of about 1 in 200, making HCM the most common disease often encountered in daily clinical practice. Understanding of HCM has progressed with advances in genetic testing, imaging, and management.
Case Report
This brief manuscript illustrates the clinical case of a 54-year-old amateur athlete who practices road cycling and mountain biking. He comes to our sports cardiology center to renew his eligibility for competitive activities. The athlete reports that for about a couple of months, he had been experiencing considerable difficulty at the start of physical activity, manifested by discomfort and chest oppression that disappeared after a few minutes, thus allowing him to continue cycling, and although lately, this has happened infrequently, it has forced him to stop physical activity. The physical examination was unremarkable, while the family history revealed presumed unspecified cardiomyopathy of a maternal aunt. The resting ECG was within normal limits (Figure 1A), while the exercise test on the cycle ergometer showed signs of significant ST-segment depression (Figure 1B) in the precordial leads in the absence of symptoms.
 Figure 1: Figure 1A showed normal ECG at rest; Figure 1B showed diffuse significant ST depression in precordial leads at the exercise ECG stress test.
Figure 1: Figure 1A showed normal ECG at rest; Figure 1B showed diffuse significant ST depression in precordial leads at the exercise ECG stress test.
The attending physician suspected a reduction in coronary reserve and therefore ordered a coronary CT scan to rule out the presence of subclinical obstructive coronary artery disease or deep myocardial bridging. However, the CCTA was completely negative and showed a free coronary tree, while a marked basal interventricular septal thickness was reported laterally. This evidence was confirmed by a subsequent 2DTT echocardiogram that showed marked and pathological hypertrophy of the basal segment of the IVS (Figure 2A) and suspected SAM (Figure 2B) of the anterior mitral leaflet, with a slight increase in the subaortic gradient during the Valsalva maneuver.
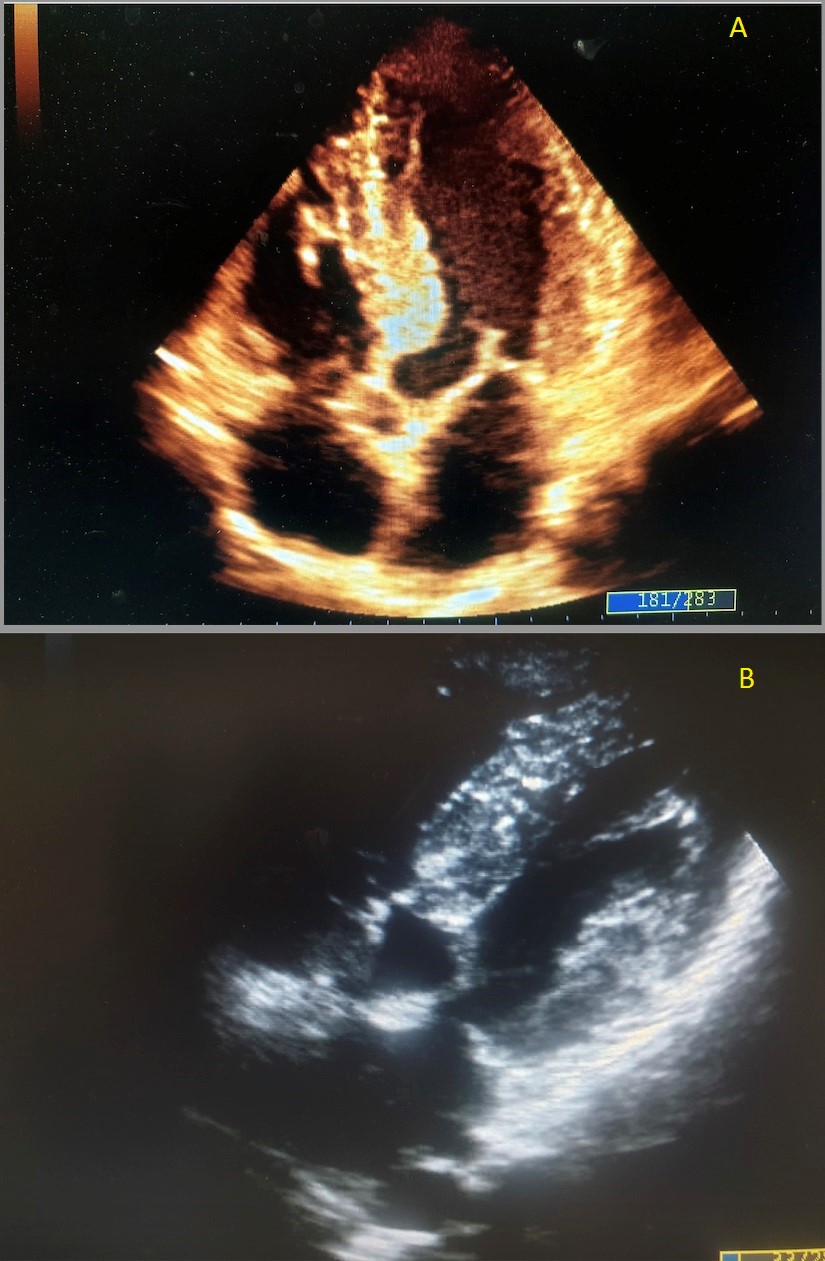 Figure 2: Figure 2A showed left ventricular hypertrophy of the basal IV septum by apical 4 chamber view; Figure 2B showed systolic
Figure 2: Figure 2A showed left ventricular hypertrophy of the basal IV septum by apical 4 chamber view; Figure 2B showed systolic
anterior motion (SAM) of the left anterior leaflet by subcostal view.
Therefore, the athlete promptly underwent cardiac stress echo, which showed dynamic LVOT obstruction by SAM at the beginning of the effort with a pathological gradient increase of more than 80 mmHg (Figure 3). A subsequent 3D ultrasound examination confirmed the presence of a marked asymmetric hypertrophy of 18 mm at the level of the basal mid-segment of the IVS and the inferior wall, with a marked reduction in global longitudinal strain (GLS). All these findings confirmed the presence of a dynamic obstructive subaortic HCM in a paucisymptomatic athlete.
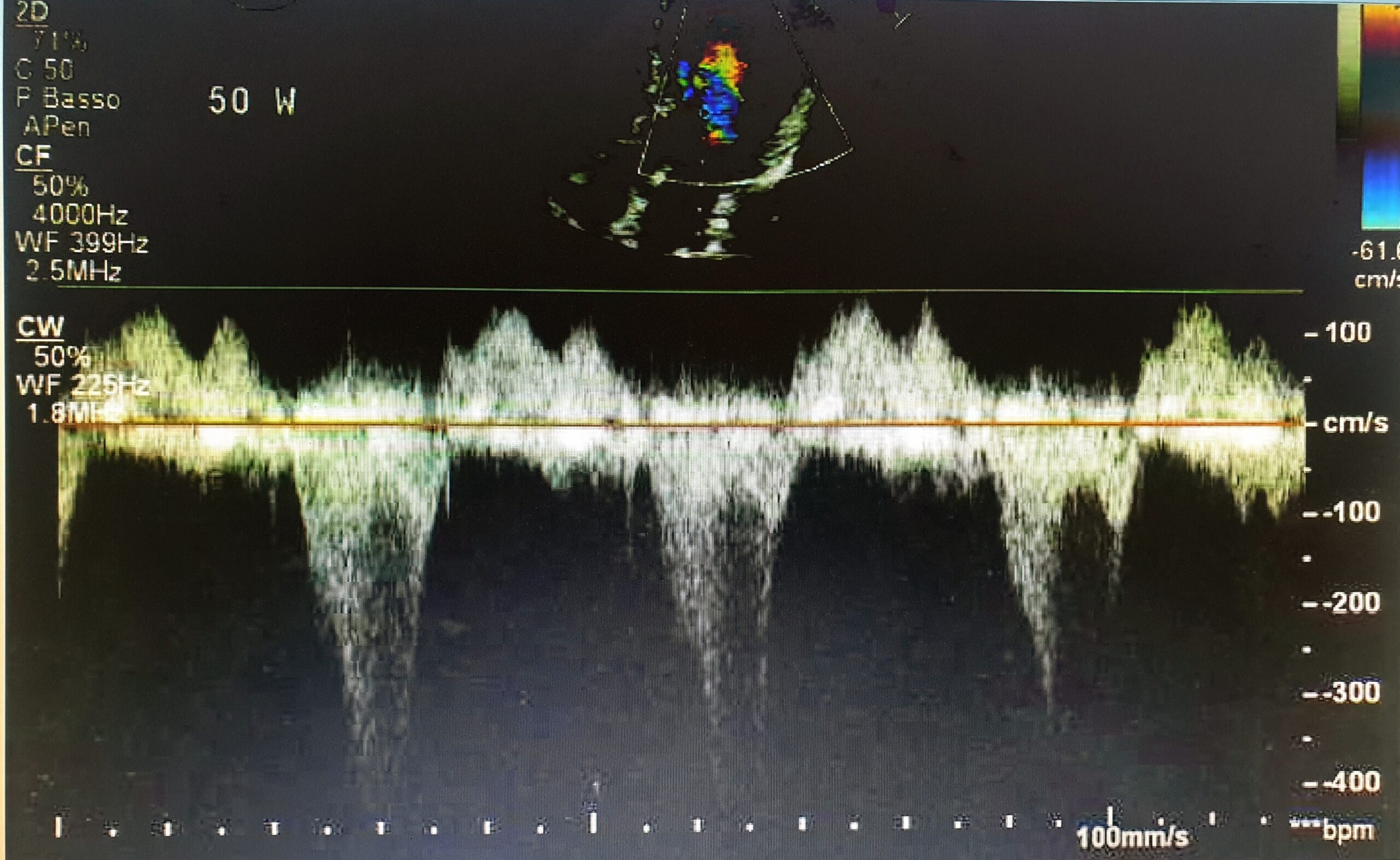 Figure 3: The figure showed dynamic LVOT obstruction by SAM with LVOT gradient over 80 mmHg.
Figure 3: The figure showed dynamic LVOT obstruction by SAM with LVOT gradient over 80 mmHg.
Discussion
Athletes who perform regular high-intensity cardio training develop a series of electrical and structural cardiac adaptations that occur functionally to improve athletic performance. About one-fifth of athletes younger than 35 show a major LV wall thickness compared to sedentary individuals. There most of them do not develop LV thickening greater than 12 mm.
However, only a small percentage of athletes develop LV parietal thickness of 13–16 mm, which overlaps with aspects of morphologically mild HCM. Unfortunately, HCM is traditionally seen as the most common condition responsible for sudden cardiac death (SCD) in athletes [9, 10].
Studies have also revealed a strong male preponderance for SCD, particularly in African-American athletes competing in sports such as soccer and basketball, both characterized by rapid, violent, and sudden high cardiac output and muscle overload, with a sudden increase in activity adrenergic [11].
Unfortunately, over 80% of affected individuals are asymptomatic prior to SCD, which often occurs during or after exercise. Screening ECG is the first tool to detect CMI in athletes. Actually, T-wave inversion (TWI) abnormality is considered the hallmark of HCM and is observed in more than 75% of athletes with HCM, according to a recent study [12] even compared to sedentary subjects with HCM. Furthermore, ST-segment depression was observed in more than half of the athletes with HCM, and a quarter of them had pathological Q waves. In more than half of the athletes with HCM, an attenuation or decrease in the pressor response during exercise can be observed, the plausible mechanisms of which are abnormal vascular tone, small vessel ischemia, and/or subaortic exercise obstruction [13]. A normal ECG does not exclude the presence of HCM but can suggest a mild manifestation of the disease. Even though CMR ability in the assessment of HCM is improving [14], especially for intramyocardial fibrous tissue or scar detection using delayed enhancement imaging, echocardiography remains the principal tool for the diagnosis and morphological characterization of HCM.
Conclusion
This case report confirms that HCM reduces the fitness of even an amateur athlete suffering from HCM, resulting in a physical limitation in practicing high-intensity sports. Despite the clinical difficulty and paucisymptomaticity of the present case, the multiple evidence of impaired diastolic function, reduced LV cavity size, dynamic obstruction of the LV outflow tract, and coronary microvascular insufficiency caused by reduced subendocardial flow due to reduced stroke volume during exercise with low peak oxygen consumption, made the difference. Differentiating physiological LVH from morphologically mild but pathological thickness due to HCM in the athlete is particularly challenging, so the clinical process must utilize a whole range of clinical tools to direct the keen mind of a willing physician to make the correct diagnosis.
Acknowledgment
I would like to thank Dr. Andrea Barbieri for his great support.
References
- Authors/Task Force members, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-779.
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2020;76(25): 3022–55.
- Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-254.
- Basavarajaiah S, Wilson M, Whyte G, et al. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: relevance to pre-participation screening. J Am Coll Cardiol. 2008;51(10):1033-39.
- Corrado D, Basso C, Rizzoli G, et al. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42(11):1959-963.
- Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199-204.
- Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064-75.
- Pelliccia A, Fagard R, Bjørnstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(14):1422-445.
- Pelliccia A, Maron BJ, Spataro A, et al. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324(5):295-301.
- Basavarajaiah S, Wilson M, Whyte G, et al. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: relevance to pre-participation screening. J Am Coll Cardiol. 2008;51(10):1033-39.
- Harmon KG, Asif IM, Klossner D, et al. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600.
- Schnell F, Riding N, O’Hanlon R, et al. Recognition and significance of pathological T-wave inversions in athletes. 2015;131(2):165-73.
- Kawasaki T, Azuma A, Kuribayashi T, et al. Vagal enhancement due to subendocardial ischemia as a cause of abnormal blood pressure response in hypertrophic cardiomyopathy. Int J Cardiol. 2008;129(1):59-64.
- Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(3):220-28.



