The most common cause of cardiogenic shock (CS) is acute myocardial infarction (AMI), which is diagnosed in approximately 5–8% of patients hospitalized for AMI and is more common in patients with acute ST-segment elevation myocardial infarction (STEMI). CS is caused by severe myocardial dysfunction, which leads to a decrease in cardiac output, hypoperfusion of the end organs, and hypoxia. Mortality in diabetic patients with AMI is high. Besides the fact that type 2 diabetes mellitus (DM2) contributes to the progression of coronary atherosclerosis, coronary pathology in this category of patients occurs against the background of a specific diabetic myocardial lesion – diabetic cardiomyopathy. Against the background of cardiomyopathy, acute heart failure is more often developed with a decrease in global myocardial contractility up to CS, which increases hospital-acquired mortality in MI by more than 15 times. The increased risk of death observed in patients with DM2 in the acute period of myocardial infarction (MI) persists for several years, and therefore, at present, in patients with the acute coronary syndrome (ACS) and diabetes, an early invasive strategy is preferable to a conservative strategy. The SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial demonstrated that in patients with CS complicating AMI, emergency revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) improved long-term survival when compared with initial intensive medical therapy. However, in the CULPRIT-SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) study, stenting of non-infarct-dependent coronary arteries in CS increases the risks of major cardiac events, as well as the number of repeated revascularizations within 30 days and 1 year. Patients with multivessel lesions, in most cases, are elderly patients (75–90 years old) who have age restrictions on taking the loading dose at the prehospital stage. Such a loading dose of clopidogrel may not be sufficient to saturate the patient in fact, despite optimal epicardial recanalization, a large proportion of patients still experience impaired reperfusion and in-stent thrombosis. A large body of evidence has been accumulated on the benefits of glycoprotein (GP) IIb-IIIa inhibitors in terms of prevention of stent thrombosis, and benefits in mortality, especially among high-risk patients, and as an upstream strategy.
cardiogenic shock, eptifibatide, diabetes mellitus, elderly, percutaneous coronary intervention
CS: cardiogenic shock; MI: myocardial infarction; AMI: acute myocardial infarction; STEMI: ST-segment elevation myocardial infarction; DM2: type 2 diabetes mellitus; PCI: percutaneous coronary intervention; ECG: electrocardiography; LV: left ventricle; LCA: left coronary artery; LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery
The article presents a clinical case of an elderly woman with prolonged type 2 diabetes mellitus (DM2) in the acute period of myocardial infarction (MI) complicated by cardiogenic shock (CS).
The patient, a woman, 83 years old, was admitted to our hospital with a diagnosis of acute circular MI, Killip-IV, CS, and DM2 for emergency coronary angiography. The patient was intubated at the prehospital stage through a probe in the emergency room, the patient received 75 mg of clopidogrel and 250 mg of aspirin according to age indications. On echocardiography, global contractility is reduced, left ventricle ejection fraction (LVEF)-22%, and diffuse hypokinesis. On the electrocardiography (ECG) large-focal changes in the anterior left ventricle (LV) wall, and lower LV wall, signs of circular MI.
On coronary angiography of the left coronary artery (LCA), the following are visualized: stenosis of the LCA trunk in the bifurcation area – 70%, occlusion of the middle third of the left anterior descending artery (LAD). Assessment of the TIMI GRADE FLOW (TIMI)-0, the state of myocardial perfusion MBG-0 (myocardial blush grade); diffuse changes with stenosis – 70–80% of the left circumflex artery (LCx) (Figure 1).
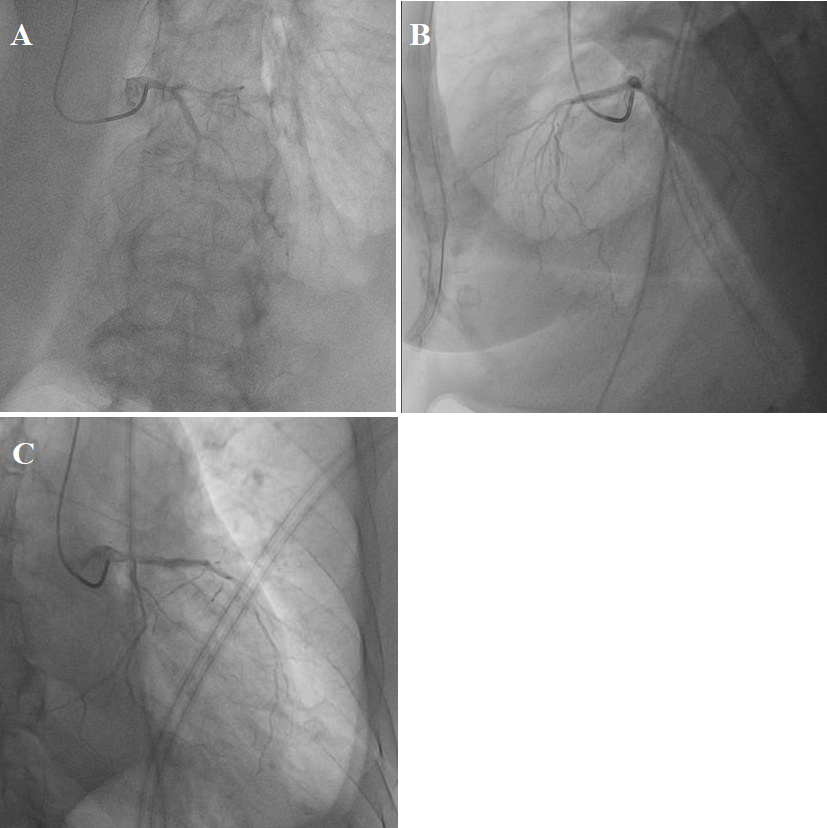
Figure 1: (A, B, C) Coronarography LCA, thrombotic occlusion LAD.
The right coronary artery (RCA) coronarography shows thrombotic occlusion of the middle third of the RCA, with slowing of blood flow to the periphery TIMI-0, MBG-0, assessment of the degree of thrombosis TTG (TIMI THROMBUS GRADE)-5 (Figure 2).
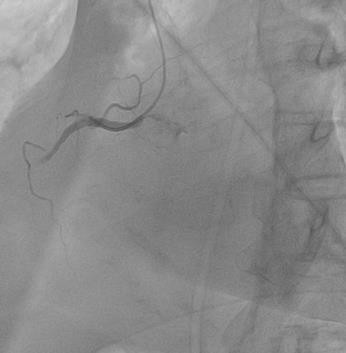
Figure 2: Coronarography RCA, thrombosis of the middle portion of the RCA.
According to the recommendations of the “European Cardiological Community” (ESC) 2018 and taking into account the complication of MI – CS, a decision was made to immediately restore blood flow in the infarct-dependent LAD and RCA. The guide catheter is installed at the ostium of the LCA trunk. The coronary wire was placed behind the occlusion of the coronary artery. Predilation was performed in the occlusion zone with a 2*15 mm balloon catheter. Two drug-coated stents (everolimus) of 3*22 m and 2.75*26 mm were implanted sequentially. The antegrade blood flow TIMI-3 was restored in the distal part of the LAD (Figure 3).
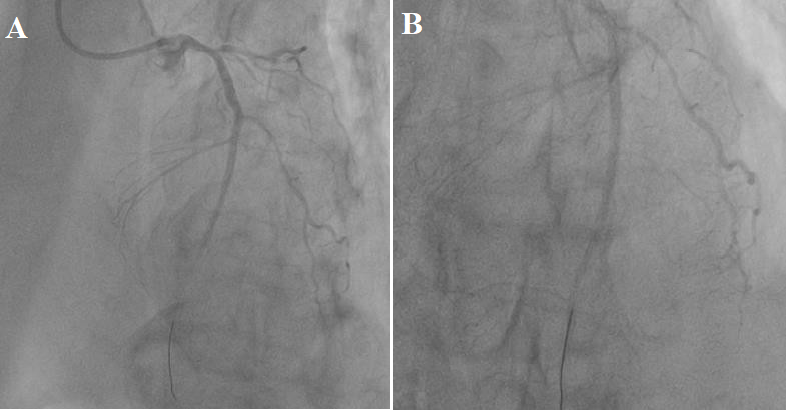
Figure 3: (A, B) Antegrade blood flow restored by TIMI-3 after implantation of 2 stents.
The next step was the treatment of RCA. The coronary wire is inserted through the guide catheter into the lumen of the artery. Angioplasty was performed on the thrombosis RCA. Blood flow to the periphery was restored TIMI-3. We have sequentially implanted 2 drug-coated stents in the RCA (Figure 4).
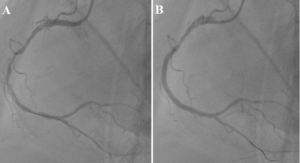
Figure 4: A – RCA after balloon angioplasty, B – RCA after stents implantation.
After the stenting of the RCA on the ECG monitor, there were changes in the leads responsible for the anterior wall of the LV in the form of ST-segment elevation. Repeated coronary angiography of LCA was performed (Figure 5).
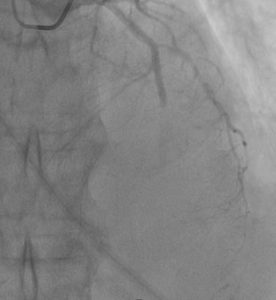
Figure 5: Acute stent thrombosis in coronary angiography of the LAD, the phenomenon of no-reflow.
The dose of eptifibatide per patient’s body weight was calculated. Through an aspiration catheter, a bolus dose of eptifibatide – 7.3 ml was injected locally into the permanent residence, and a total solution of nitroglycerin at a dose of 200 mcg was administered intracoronary. On the control coronary angiography, blood flow was restored along the LAD and branches of TIMI-3, and MBG-2 (Figure 6).
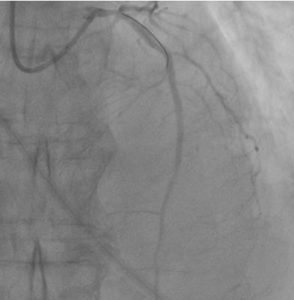
Figure 6: Control coronary angiography after intracoronary eptifibatide administration. Regression of the no-reflow phenomenon. Blood flow of LAD TIMI-3, MBG-2.
Stenting of the LCA trunk and LCx was postponed in accordance with the ESC 2018 recommendations on myocardial revascularization. A second dose of eptifibatide was administered intravenously by bolus and an infusion was established at a rate of 6.5 ml per hour. Through the right transfemoral access, an intra-aortic balloon counterpulsation (IABC) is established under the control of an echocardiographic sensor in the ratio of heart rate 1:1. Through an esophageal probe, the patient received a loading dose of ticagrelor 180 mg. After 4 days, the patient was extubated, with tracheostomy and continued ventilation. On the 12th day, the patient was transferred to the emergency cardiology department. Echo control showed an improvement in the contractility of the myocardium up to 44%. The patient was transferred to the hospital for further rehabilitation.
Based on the CULPRIT-SHOCK study, it is safer to stent only the infarct-dependent artery in STEMI with CS. The choice of revascularization tactics in patients with STEMI complicated by CS and with a comorbid background (for example diabetes mellitus) should be discussed collectively in the cardiovascular team. The cardiovascular team determines the indications for stenting of non-infarct-dependent arteries and mechanical support. The introduction of platelet glycoprotein receptor IIb/IIIa inhibitors through an intracoronary perfusion catheter selectively into the thrombosis zone in the AMI infusion study was associated with a statistically significant decrease in the size of the infarction by 2% in magnetic resonance imaging (MRI) [1–9], and the AIDA STEMI intracoronary infusion study was associated with a decrease in the incidence of heart failure [10].
Thus, the decision on the introduction of an antiplatelet agent, emergency revascularization (complete or partial), and methods of mechanical or medical support for each patient with ST-segment elevation MI complicated by CS is determined individually by the cardiovascular team based on the recommendations of the European Society of Cardiology.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data supporting Figure1–6 are not publicly available in order to protect patient privacy.
The authors declare that there is no conflict of interest.






