Sarcocystosis is a critical parasitic zoonosis caused by Sarcocystis species, an intracellular protozoan parasite of the Apicomplexa phylum and one of the most prevalent parasitic diseases among wild and domestic animals all around the world. Infection in the definitive host is mainly characterized by the formation of cysts in muscle tissue. In intermediate host skeletal muscles, the diaphragm and heart are the favored locations for Sarcocystis spp. While we were examining the heart of a three-month-old dead lamb, we incidentally observed striking, white, and discrete spots, measuring 2–3 mm, that were diffusely distributed in the endocardium. Microscopically, numerous Sarcocystis were seen within cardiomyocytes and Purkinje fibers. No different pathological modifications had been found in inflamed muscle fibers or the surrounding interstitium. To the best of our knowledge, there is no case report about diffuse involvement of endocardium by Sarcocystis spp., and this unique form of sarcocystosis prompted us to place the current case on record.
endocardial sarcocystosis, lamb, Iran
Sarcocystosis is a significant zoonotic disease caused by Apicomplexan protozoa [1, 2]. Infections by Sarcocystis can result in financial losses in animal farming, threatening food safety, and a public health issue [3]. The parasites typically develop in a heteroxenous predator-prey life-cycle involving final (carnivore) and intermediate (omnivore/herbivore) hosts [4]. Sarcocystis infections of sheep are common all around the world. The four most important species of Sarcocystis; (S. arieticanis, S. gigantea, S. medusiformis, and S. tenella) have been detected in sheep [5]. Of these, S. tenella and S. arieticanis are pathogenic species that form microscopic cysts and are transmitted via canids, whereas S. gigantea and S. medusiformis, non-pathogenic species, form macroscopic cysts and are transmitted via felids [6, 7]. S. tenella, S. arieticanis, and S. gigantea are spread globally, including in Iran. Though, S. medusiformis has only been reported in European and Asian nations [8]. Sarcocystosis causes weight reduction, untimely birth, fetus removal, and even passing in sheep; these animals are typically infected by drinking water and hunting for feed contaminated with Sarcocystis sporocysts [9]. In the intermediate hosts, the sarcocysts are often found in the heart, tongue, esophagus, diaphragm, skeletal muscle, and rarely in the central nervous system [10]. This study aims to report an unusual form of diffuse endocardial sarcocystosis. To the best of our knowledge, there is no similar case in the world.
A three-month-old dead lamb was referred to the Veterinary Clinic at Shahrekord University, Shahrekord, Iran, with a history of mild fever, reduced feed intake for a few days, and sudden death. An autopsy of the carcass was performed, and gross pathologic lesions were registered. The carcass showed poor body condition, anemia, and pale and icteric mucosal membranes on necropsy examination. While examining the lamb’s heart, we observed striking, white, and discrete spots, measuring 2–3 mm, that were diffusely distributed in the endocardium (Figure 1).
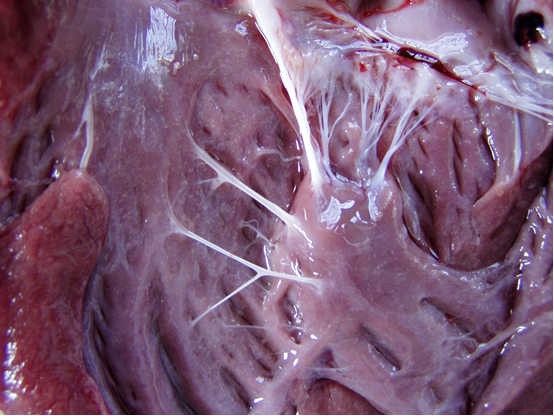
Figure 1: Note white spots in the opened left ventricle.
Mild pulmonary edema and congestion were also observed; other organs were normal. For histopathological examination of the spots on the heart, samples were taken and fixed in 10 percent buffered formalin and processed according to the standard histological techniques for paraffin embedding. Sections of 5 μm thickness were cut and stained with hematoxylin & eosin and then studied [11]. Microscopically, numerous thin-walled sarcocysts were seen within cardiomyocytes and Purkinje fibers (Figure 2).
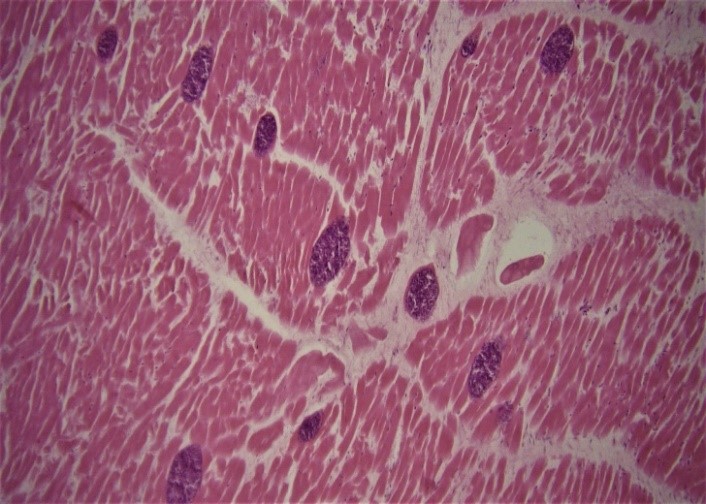
Figure 2: Severe cardiac sarcocystosis. The sections of sarcocysts are observed within cardiomyocytes (Hematoxylin and Eosin, × 10).
The sarcocysts were full of basophilic bradyzoites that appeared as round, elongated, or crescent-like bodies depending on the cutting plane (Figure 3). No different pathological modifications had been found in inflamed muscle fibers or the surrounding interstitium.
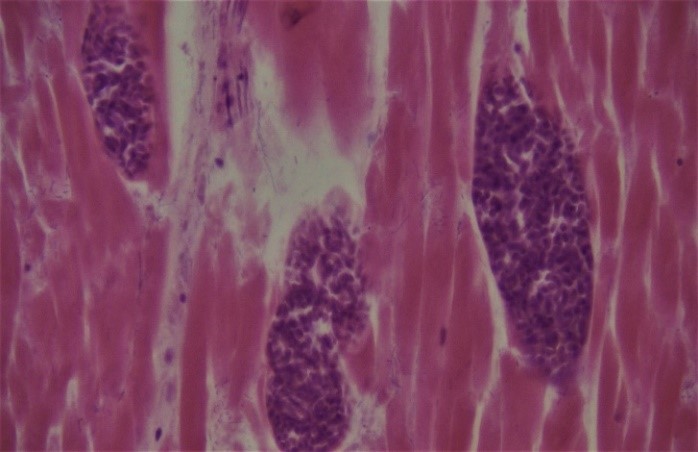
Figure 3: Higher magnification of three sarcocysts that basophilic bradyzoites are seen within the cysts (Hematoxylin and Eosin, × 40).
Muscular sarcocystosis is acquired by eating infected oocysts from the definitive host. Each oocyst releases four sporozoites, which invade the intestinal mucosa and transform into schizonts in the capillary endothelium. The first asexual reproduction occurs in the endothelium of small vessels of the mesenteric lymph nodes. Subsequent hematogenous spread results in merozoites entering the microcirculation of muscle tissue of intermediate hosts harboring sarcocysts or zoitocysts in their muscles [12]. Muscular sarcocystosis causes a broad spectrum of clinical manifestations, including myositis, myalgia, localized painful muscular swelling, low-grade fever, weakness, vasculitis, and eosinophilia [9, 13–15]. To the best of our knowledge, there is no report about diffuse involvement of endocardium by Sarcocystis spp. In intermediate hosts, sarcocysts are often found in the skeletal muscle, tongue, esophagus, diaphragm, and heart, and rarely in the central nervous system and gut [10, 16]. Still, these infections are not massive and diffuse. Sheep are the intermediate hosts of at least six species of Sarcocystis, including S. gigantea (syn. S. ovifelis), S. tenella (syn. S. ovicanis), S. arieticanis, S. medusiformis, S. microps, and S. mihoensis [17]. All species are morphologically distinguished based on their sarcocyst wall ultrastructure [18]. Electron microscopy and molecular methods must be used to identify Sarcocystis species [19]. In this study, Sarcocystis species was not identified. Still, the sarcocysts were macro-size, so it could be S. gigantea or S. medusiformis because these species produce macroscopic cysts and are transmitted by felids. In contrast, S. arieticanis and S. tenella have microscopic sarcocysts shared by canids [20].
Based on the results of this study, sarcocystosis should be taken as a differential diagnosis in cases with diffuse white-spotted endocardial lesions.
Conceptualization: [Hossein Nourani]; Methodology: [Hossein Nourani/Soheil Sadr]; Formal analysis and investigation: [Hossein Nourani]; Writing – original draft preparation: [Hossein Nourani/Soheil Sadr]; Writing – review and editing: [Hossein Nourani/Soheil Sadr]; Supervision: [Hossein Nourani]
No funding was received for conducting this study.
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
The authors declare no conflict of interest.
The authors thank Mr. Ahmadi for their assistance in preparing the specimens.



