Incessant ectopic atrial tachycardia (EAT) is well-known in the electrophysiology world. However, once accompanied by neurological manifestations, hence is a dilemma. We are reporting a 26-year-old male presenting with incessant symptomatic EAT. Once cardioverted, he began to manifest cardiovascular stroke clinical symptoms. Further objective investigation revealed no evidence of cardioembolic stroke. Our aim of this case report is to explore if there is a true correlation between EAT and possible neurological association and open further guidelines related to research.
ectopic atrial tachycardia, supraventricular tachycardia, heart rate, heart disease
EAT: ectopic atrial tachycardia; SVT: supraventricular tachycardia; AT: atrial tachycardia; bpm: beats per min; ECG: electrocardiogram; EAR: ectopic atrial rhythm; CT: computed tomography; TEE: transesophageal echocardiogram; PFO: patent foramen ovale; AF: atrial fibrillation; ESVEA: excessive supraventricular ectopic activity
Ectopic atrial tachycardia (EAT) is defined as the development of ectopic focus in one of the atria that conduct impulses at a higher rate than the sinoatrial node. This is attributed to abnormal automaticity, micro-reentry, or triggered activity taking place in the ectopic focus [1]. EAT accounts for approximately 10% of the overall incidence of supraventricular tachycardia (SVT) [2]. The heart rate in atrial tachycardia (AT) ranges from 130–250 beats per min (bpm) and may reach up to 300 bpm. It is characterized by P waves whose morphology differs from the P wave morphology during sinus rhythm [3]. Over long-term follow-up, spontaneous remissions of EAT were frequent. In young children, the rate of spontaneous resolution accounts for about ~50% during the first year of life [4]. This may explain the rarity of adult EAT diagnoses. The higher rate of spontaneous remission among younger patients suggests that rhythm disorders in younger patients may be a result of immature cardiac tissue or an inflammatory process followed by subsequent healing [5]. On the other hand, in older patients, EAT may be caused by a local degenerative process that does not regress and so does not allow spontaneous remission and is difficult to control [5]. A follow-up electrocardiogram (ECG) on patients diagnosed with EAT 3–16 years previously revealed that 26% of patients still had an ectopic atrial rhythm (EAR) [6]. EAR is defined as a regular atrial rhythm that is more than 100 bpm. The prevalence of EAR is 1.13% and increases with age [7]. Patients with AT might be asymptomatic or typically present with palpitations, chest pain or pressure, lightheadedness, or shortness of breath [3]. Underlying heart disease is a well-known risk factor, however, a histological analysis conducted on the myocardium from AT focuses revealed normal findings in the majority of patients [1]. Diagnosis can be challenging in patients who present with paroxysmal AT and may require Holter monitoring or a loop recorder [8]. We hereby report a case of incessant non-sustained EAT in a 26-year-old man who was admitted for chest tightness and palpitations, followed by atypical presentation with numbness in the left face, arm, and leg.
A 26-year-old male, not known to have any chronic medical illness nor a family history of heart disease or sudden death presented to the emergency department complaining of left-sided chest pain associated with shortness of breath and palpitations. The patient has had these symptoms intermittently over the past year, but for 2 weeks, symptoms occur daily. Physical examination was notable for intermittent chest tenderness. The patient was vitally stable (a pulse rate of 90 bpm, regular, normal blood pressure, respiratory rate, and oxygen saturation of 100% on room air).
The ECG findings on admission showed incessant non-sustained AT (Figure 1). An echocardiogram (ECHO) showed a normal study with an estimated ejection fraction of 55% and unremarkable chest X-ray findings. An initial laboratory study showed normal results of a complete blood count, liver function tests, basic screening profile, renal profile, thyroid function test, and D-dimer.
The patient was admitted to the cardiac care unit for close monitoring of serial ECGs and troponin levels with observation for any dysrhythmia. He was started on bisoprolol 2.5 mg per oral, and magnesium sulfate with frequent follow-up of electrolyte. On the next day, the patient complained of persistent chest pain with palpitations; ECG was unchanged, indicating no response to bisoprolol. In view of his unresponsiveness to β-blockers, he was shifted to non-dihydropyridine calcium channel blockers (verapamil 40 mg three times daily). Two days later, in the advent of unresponsiveness to neither atrioventricular blocking agents, flecainide was initiated in an attempt for pharmacological cardioversion. This decision was imperative in a setting of normal cardiac structure evident by a normal echocardiogram.
After maintenance of his sinus rhythm with flecainide (Figure 2), the patient reported episodes of numbness in his tongue and mandible with mild dizziness. The numbness started in the left face, then progressed to the left arm and leg. He was referred to neurology in-patient service, and on physical examination, the patient appeared to be in no distress, fully conscious, alert, and oriented to person, place, and time. No dysarthria or neurological deficits were recognized. Intracranial hemorrhage and infarction were ruled out by brain computed tomography (CT) with no evidence of structural abnormality on magnetic resonance imaging (MRI).
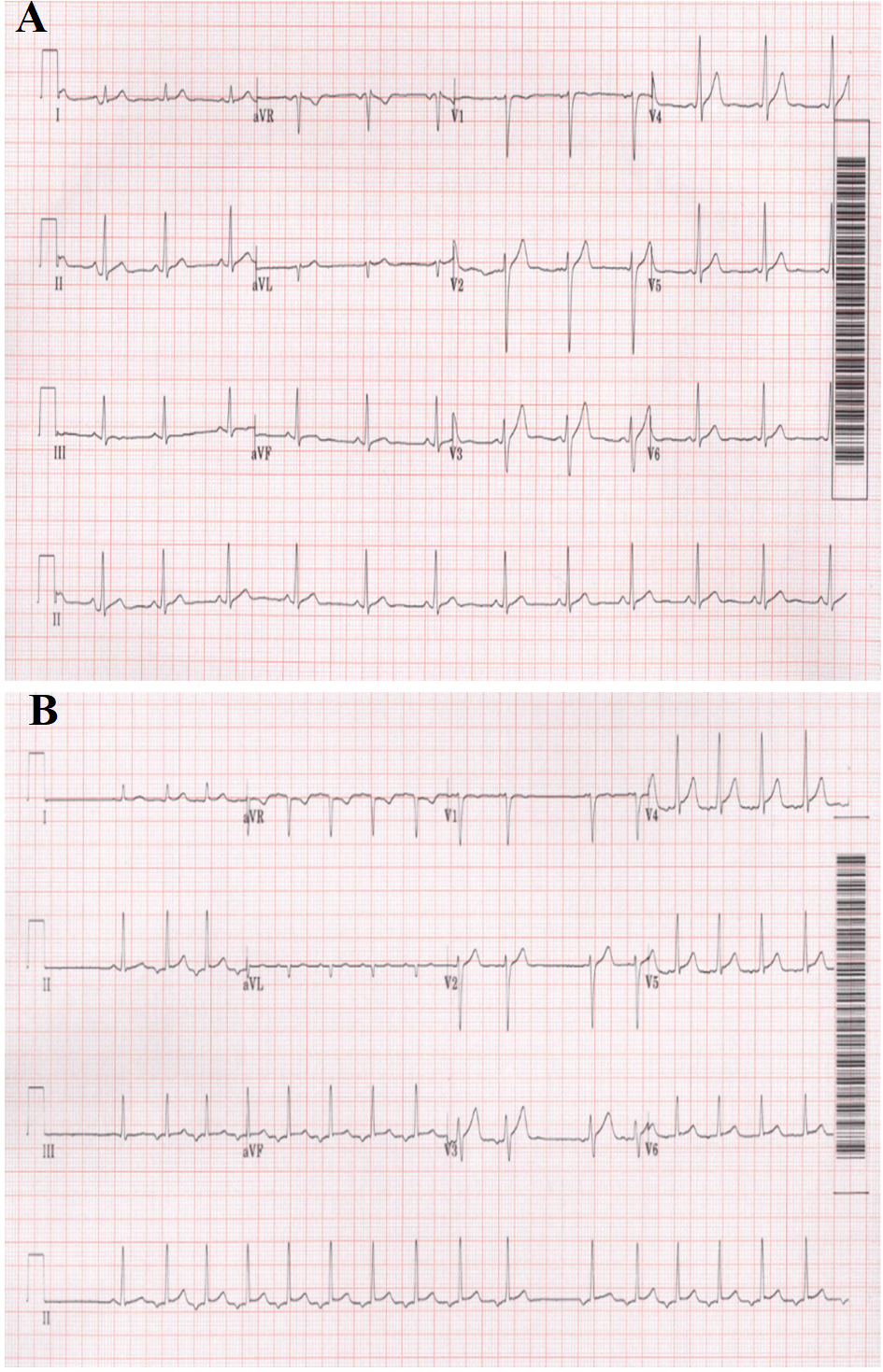 Figure 1: ECG shows alternating rhythm between normal sinus rhythm (A) and ectopic atrial rhythm with recognizable ectopic atrial tachycardia (B); the P wave is negative in leads Ⅱ, Ⅲ, aVF, and isoelectric in lead V1.
Figure 1: ECG shows alternating rhythm between normal sinus rhythm (A) and ectopic atrial rhythm with recognizable ectopic atrial tachycardia (B); the P wave is negative in leads Ⅱ, Ⅲ, aVF, and isoelectric in lead V1.
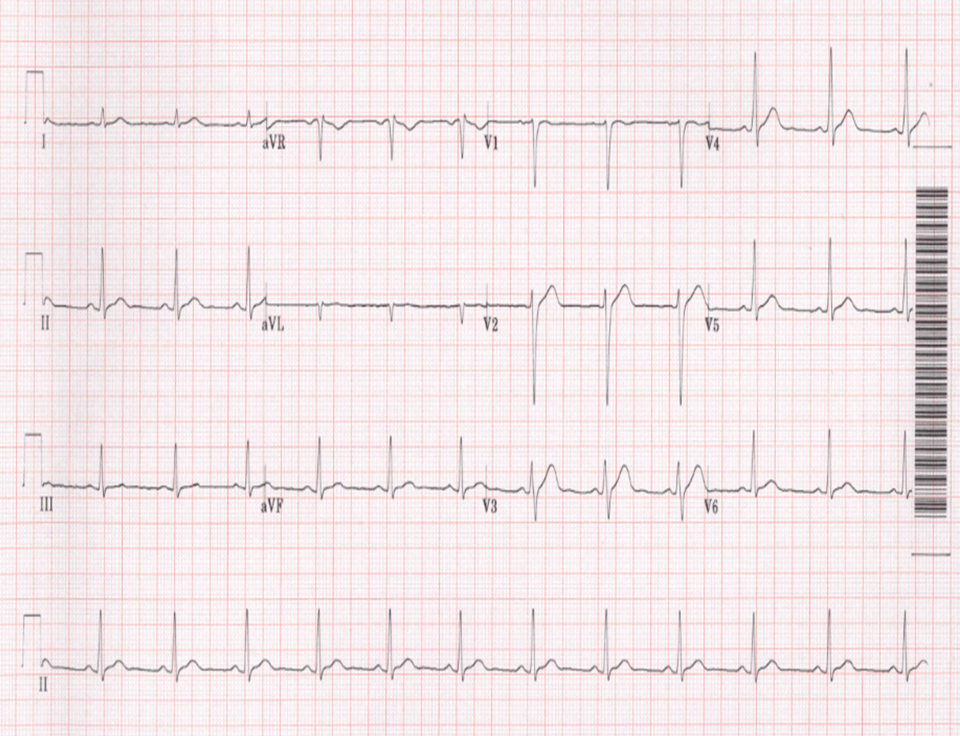
Figure 2: ECG shows maintenance of sinus rhythm after administration of flecainide.
The next day, an ECG tracing revealed normal sinus rhythm with intermittent EAT (Figure 3). Cardiac CT coronary angiography was done and revealed no obstructive coronary artery disease or anomaly. The patient continued on flecainide in an escalating dose of 50 mg twice daily, then 100 mg twice daily, and apixaban 5 mg twice daily was added in fear of transient ischemic attacks. Transesophageal echocardiogram (TEE) revealed no intracavitary thrombi, however, it was reported positive for a patent foramen ovale (PFO).
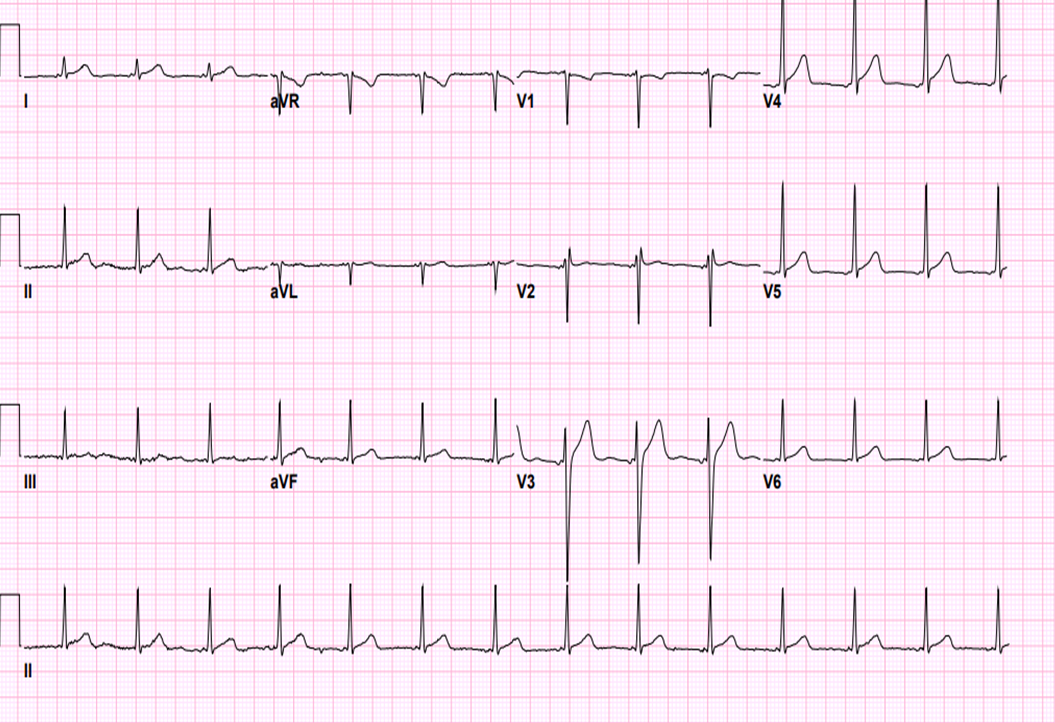 Figure 3: ECG after successful left side atrial tachycardia ablation, the P wave morphology has changed; in lead aVL the P wave is positive, isoelectric in leads Ⅱ, Ⅲ, aVF.
Figure 3: ECG after successful left side atrial tachycardia ablation, the P wave morphology has changed; in lead aVL the P wave is positive, isoelectric in leads Ⅱ, Ⅲ, aVF.
Eventually, the patient underwent successful catheter ablation of AT originating from the anterior inferior interatrial septum, targeted from the left side via a trans-septal approach (Figure 4). Immediately after ablation, the patient converted to sinus rhythm with a short PR interval may be indicating partial affection of the Ganglionic Plexus supplying the AV node (located nearby the site of origin of AT). He was then scheduled for regular outpatient clinic follow-up visits.
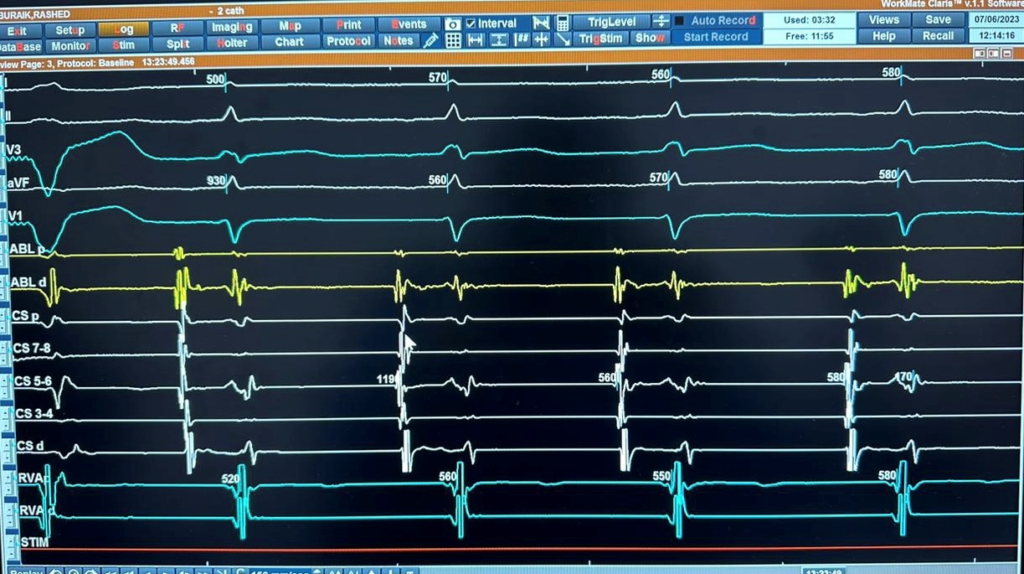 Figure 4A: Intracardiac recording showing the earliest atrial activation at CS 5,6.
Figure 4A: Intracardiac recording showing the earliest atrial activation at CS 5,6.
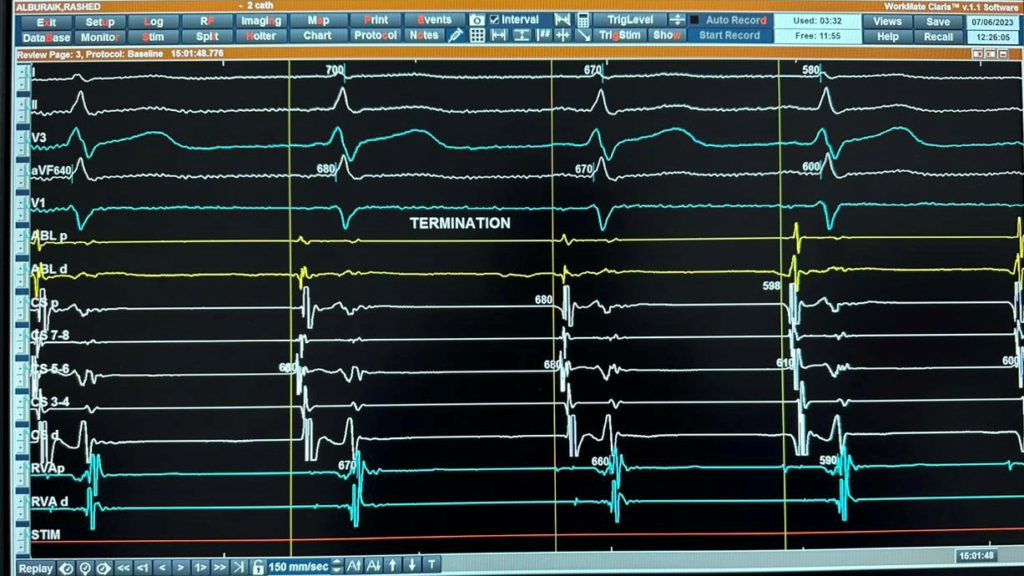 Figure 4B: Intracardiac recordings of AT during ablation showing termination of AT. C 5,6 not the earliest.
Figure 4B: Intracardiac recordings of AT during ablation showing termination of AT. C 5,6 not the earliest.
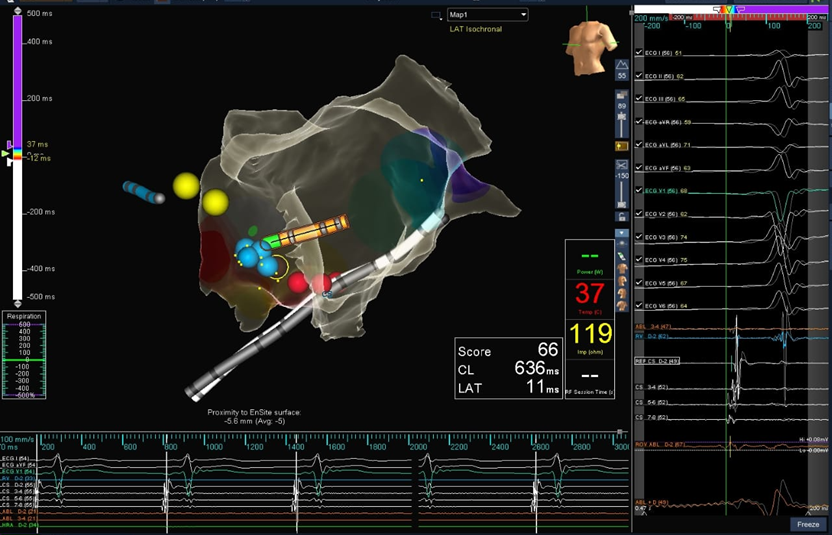
Figure 4C: 3D mapping for geometry and voltage showing the successful site of ablation – left side lower part of the anterior interatrial septum.
EAT is a type of SVT that arises from an ectopic focus in the atria. It is characterized by a rapid, regular atrial rhythm with a rate of 130–250 bpm [9]. Even though EAT may present at any age, it is predominantly seen in young infants and children [10]. As well as children, adult cases have been documented [11]. An incessant tachycardia accompanied by palpitations is the most common presentation. Focal AT usually runs a benign course; however, if incessant AT persists, it may lead to tachycardia-induced cardiomyopathy [3, 12]. Tachycardiomyopathy (TCM) is a form of dilated cardiomyopathy and systolic dysfunction caused by chronic arrhythmia and is associated with a high risk for sudden cardiac death [13]. Despite this, it can also be asymptomatic and remain undiagnosed. A retrospective study conducted by Poutiainen et al. [6] showed that 0.34% of asymptomatic young subjects and 0.46% of symptomatic patients had ECG evidence of EAT.
Little is known about the etiology of the disease. In children, EAT is caused by the relatively immature myocardium and electrophysiologic characteristics of conduction tissue [14, 15]. It has been found in healthy hearts as well as in patients with congenital heart disease, post-cardiac surgery, myocarditis, bronchopulmonary dysplasia, severe pulmonary hypertension, and hypertrophic cardiomyopathy [16]. In adults, EAT is primarily caused by diseased atrial myocardium and local degenerative processes that do not regress [17]. Also, it has been observed with acute drug intoxication (for example, digitalis, amphetamines, and tricyclic antidepressants), acute and chronic heart disease, obstructive pulmonary disease, acute infection, and a variety of metabolic disorders [11].
Our patient, whose previously healthy, was presented with intermittent episodes of tachycardia over 1 year, which was not attributed to a personal or family history. ECG showed characteristic features of an EAT, including alternating rhythm between normal sinus rhythm and ectopic atrial focus (regular narrow QRS complex tachycardia with an abnormal P-wave morphology) (Figure 1). Although it can be difficult to differentiate atrial origins close to the sinus node, it usually differs from the P-wave morphology in sinus rhythm. A TEE later revealed an incidental finding of PFO, which may be contributing to our patient’s EAT.
The management of EAT can be challenging to some extent as the clinical course of EAT differs between young children and those 3 years and older [18], with poor response to antiarrhythmic drugs among adults [17]. Treatment strategies for EAT depend on several factors and include [9]:
- Observation: In asymptomatic patients or if it’s infrequent and self-limiting, monitoring may be sufficient without any specific treatment.
- Medications: Antiarrhythmic medications such as digoxin, beta-blockers, calcium channel blockers, adenosine, and class IC medications such as flecainide and propafenone are used to control the heart rate and rhythm in individuals with symptomatic EAT. AT from triggered activity (most frequently found in the setting of digitalis toxicity) is sensitive to verapamil, beta-blockers, and adenosine. Verapamil alone or in combination with a beta-blocker may be effective for controlling tachycardia. Beta-blockers may be used to suppress AT due to enhanced automaticity. However, overall success rates are low. The use of amiodarone and sotalol (class III) to slow myocardial conduction and AV conduction has had modest success. Antiarrhythmic medications in class IA, such as procainamide, decrease automaticity, prolong refractoriness, and slow conduction velocity. There is generally a high rate of effectiveness (up to 75%) with antiarrhythmic agents. In patients with decreased LV function, medications must be used with caution.
- Catheter ablation: It is minimally invasive, but in most cases, radiofrequency ablation will be effective up to a degree of 95–100%.
- Cardioversion: Used if medications are not effective or if the patient is unstable.
- Surgery: Could be considered in rare refractory cases or multiple ectopic foci; options include atrial resection or maze procedure.
We used β-blockers as the first-line agent, followed by non-dihydropyridine calcium channel blockers, both of which had no effect on tachycardia in the reported case. Next, flecainide was initiated in an attempt for pharmacological cardioversion, and ECG cardioverted to normal sinus rhythm successfully. Surprisingly, the next day’s ECG tracing showed intermittent EAT. Eventually, the patient underwent successful left-side AT ablation after withholding flecainide for 24 h.
In younger children, it is mostly treated with antiarrhythmic drugs with a rhythm control rate of up to 91%, but a substantial proportion of patients still require a combination of two or more antiarrhythmic drugs in order to achieve a satisfactory outcome. Children aged 3 or older and adults are more likely to fail antiarrhythmic medication regimes, making early radiofrequency ablation a reasonable approach [18]. A study by Balla et al. to evaluate the long-term follow-up in patients who underwent RF ablation has proven EAT ablation’s safety and high acute success rate in a group of children, adolescents, and young adults. As a result, catheter ablation of EAT can be considered early in the course of these patients’ treatment. There were no serious complications in any of the patients. However, the recurrence rate was 20% at a median follow-up (38 months), which was attributed to the use of conventional mapping [19].
Ivabradine is a class of medication that is used to lower heart rate by selectively inhibiting the sinoatrial node. It has been shown to be effective in maintaining sinus rhythm and reversing left ventricular dysfunction in patients with incessant automatic AT-induced cardiomyopathy [20].
Ivabradine may be beneficial for patients with incessant automatic focal AT resistant to conventional management. This will control the rhythm and improve the LV function prior to the scheduled catheter ablation. Two cases of young adults who presented with resistant AT responded well to ivabradine with a significant reduction in heart rate and improvement in symptoms. Both cases were later successfully ablated, and both maintained sinus rhythm and are symptom-free 2 years post-procedure [20].
Several factors influence the choice of medication for EAT treatment, including underlying medical conditions, symptoms, heart abnormalities, and the mechanism of the AT. Choosing ivabradine is worth considering in cases of automatic focus, especially when flecainide could not be used.
An interesting finding in our case was when the patient developed transient neurological manifestations, including numbness that started in the left face, then progressed to the left arm and leg. The patient went thorough examination and investigation, including CT angiography, to rule out any stroke or transient ischemic attacks, all of which showed no abnormalities. However, the patient was started on apixaban 5 mg twice daily, in fear of transient ischemic attacks.
The relationship between episodic AT and the incidence of stroke is still debatable. Furthermore, it remains unclear whether a brief episode of AT induces the progression of atrial fibrillation (AF) [21, 22]. In our case, the patient presented with symptoms suggestive of stroke; however, thorough examination and investigation revealed no abnormalities. According to Larsen et al., a total of 678 patients were included in a study with a 15-year follow-up, showing patients with excessive supraventricular ectopic activity (ESVEA) are at greater risk of ischemic stroke. In addition, excessive atrial ectopy and short atrial runs had a strong correlation with ischemic stroke beyond AF [22]. Studies have also demonstrated the progressive increase of stroke rates with increasing CHA2DS2-VASc (congestive heart failure, hypertension, age 75 years or older, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, age 65 to 74 years, female) score and the risk further increases in patients with ESVEA [21, 22].
It is important to note that the risk of stroke associated with EAT may vary depending on the individual and the specific characteristics of the arrhythmia. Patients with EAT should be monitored closely for any potential neurological symptoms and may require additional evaluation or treatment if a stroke risk is suspected.
It is important to note that the potential symptoms and treatment options for EAT may vary depending on the individual and the specific characteristics of the arrhythmia. The use of other drugs, such as class I or class III antiarrhythmic agents or radiofrequency ablation, could be an alternative treatment for EAT patients who have an inadequate response to beta-blocking agents. It is important to monitor patients with EAT for symptoms of neurological dysfunction, and if stroke risk is suspected, additional assessment or treatment may be required.
The study was approved by King Abdullah International Medical Research Center (KAIMRC), reference number NRA23A/043/07, IRB/1928/23.
![]() 1, M Alshaikh Hussain
1, M Alshaikh Hussain![]() 2, AlMusaad A3,4, Al muaiweed R1, Aljohani S1, and AlTaweel M
2, AlMusaad A3,4, Al muaiweed R1, Aljohani S1, and AlTaweel M![]() *4,5
*4,5




 Figure 4B: Intracardiac recordings of AT during ablation showing termination of AT. C 5,6 not the earliest.
Figure 4B: Intracardiac recordings of AT during ablation showing termination of AT. C 5,6 not the earliest.