Diabetes mellitus (DM) is a chronic condition emerging as a significant global health concern, with more than half a billion people with diabetes mellitus (PwDM) worldwide [1]. The prevalence of diabetes has shown a consistent upward trend in India, contributing significantly to the overall global burden of the disease [2].
The National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS) in India recommends community assessment, screening, referral, and follow-up for selected noncommunicable diseases (NCDs), including diabetes, among all women and men aged 30 years and above. The program enables opportunistic screening for common non-communicable diseases at NCD clinics within the district hospital (DH) and Community Health Centre (CHC) [3]. However, due to the large number of PwDM and restrained logistics, it is challenging to implement a specific screening program for diabetes complications in India [4].
Diabetic retinopathy (DR) has emerged as the most common cause of preventable vision impairment (VI) and blindness on a global scale, representing a significant consequence of diabetes [5, 6]. Diabetic macular edema (DMO) and proliferative diabetic retinopathy (PDR) are the two most common causes of VI in sight-threatening diabetic retinopathy (STDR) [4]. Early screening and prompt referral for treatment is recommended before it progresses into STDR [6]. At least one-fifth of PwDM have some form of DR [7] and, as recommended, should have at least one annual dilated eye examination [8]. However, only one-third of people with diabetes undergo eye examinations [5], and only 60% receive some treatment in India [9].
Different screening modalities (e.g., direct and indirect ophthalmoscopy, stereoscopic seven-field fundus photography, nonmydriatic and mydriatic fundus photography) are used to screen DR [6]. However, seven fields of stereoscopic fundus photography remain the gold standard for DR detection [10]. Meanwhile, a digital fundus camera with a minimum image resolution of 2–3 megapixels has been accepted globally to be sufficient to display a single microaneurysm (an early sign of DR) [11].
The systematic implementation of a comprehensive DR screening program remains limited in numerous countries, including India [11]. This is mainly due to the scarcity of a trained workforce, logistical difficulties due to involved costs, and noncoverage by health insurance [11]. Pandit et al. [12] have suggested that a screening test for DR should possess a minimum specificity of 80% and a sensitivity of 95% compared to a reference standard (human grader). Conversely, other studies have demonstrated a sensitivity of 91.7% (95% CI: 91.3-92.1%) and a specificity of 91.5% (95% CI: 91.2-91.7%) for a DR screening program [13].
The utilization of telemedicine for DR screening has proven cost-effective, particularly for patients residing in rural regions that face constraints in accessing transportation services [14]. Various studies have been conducted in India to assess the effectiveness of different modes of DR screening, particularly hospital-based, community-based, and telemedicine-based screening [15]. However, it is challenging to implement a single DR screening model, given the large number of PwDM to be screened and the scarce resources for a screening program [15]. Artificial intelligence (AI) and machine learning (ML) algorithms for evaluating retinal pictures is a potentially cost-effective approach, considering the scarcity of ophthalmologists and optometrists [16]. The available evidence from India reported that an AI algorithm produced a sensitivity of 96% and a specificity of 80% in detecting any DR and a sensitivity of 99% and a specificity of 80% in detecting STDR [17].
Despite the supporting evidence of the effectiveness of DR screening programs, there is a significant gap between their widespread implementation, integration into primary healthcare settings, and accessibility of these interventions [18, 19]. The absence of sufficient health literacy and awareness regarding eye examinations and inadequacies in the referral system hinder individuals from accessing and utilizing these screening services [19].
It is also essential to assess the impact of screening programs on vision outcomes to make well-informed decisions on resource allocation toward the most effective screening programs [20]. The implementation outcomes of the screening programs can be assessed at several levels, including the individual, organizational, and population levels [21–23].
The RE-AIM framework assesses Reach (R), Effectiveness (E), Adoption (A), Implementation (I), and Maintenance (M), has emerged as an early outcome-oriented approach to address individual and organizational factors [24]. The RE-AIM planning and evaluation framework is widely used and cited in public health interventions [25, 26]. The RE-AIM framework pragmatically enhances [23, 24] the generalizability and translation of evidence-based interventions into sustained services [24, 27].
Guided by the RE-AIM framework, this protocol paper compares implementing different DR screening models in primary healthcare settings. It explores the stakeholder perspective on accessing DR screening and the cost-effectiveness of the two DR screening strategies.
Study aim and objectives
To assess the feasibility of implementing different DR screening models in primary health care settings in northern India.
The following objectives will meet this aim:
- To assess and compare the feasibility of DR screening models: 1) fundus image capture and grading by non-ophthalmologists 2) fundus image capture and grading by AI vs. 3) standard care in a rural block of Northern India.
- To understand multiple stakeholders’ perspectives on the assets and barriers to accessing DR screening in primary healthcare health facilities in the rural block of Northern India.
- To estimate the cost-effectiveness of implementing DR screening models, 1) fundus image capture and grading by non-ophthalmologists, 2) fundus image capture by non-ophthalmologists and grading by AI, and 3) standard care in a rural block of Northern India.
Application of the RE-AIM framework for comparison of two DR screening models
The primary objective of employing the RE-AIM framework is to augment the practicality of interventions, hence facilitating the planning, execution, documentation, and selection of interventions for large-scale implementation. The components of the RE-AIM framework include the following: (1) reach (percentage and representativeness of individuals who receive an intervention; (2) effectiveness (impact of an intervention on outcomes); (3) adoption (proportion and representativeness of settings and providers willing to deliver the intervention); (4) implementation (the extent to which the intervention is implemented as intended); and (5) maintenance (sustainability of the intervention at the individual and setting levels) (Supplementary Figure 1).
It explains identifying the population to be reached and benefited by the interventions (PwDM). The recruitment pool will primarily be defined as the total population of all age groups in the villages served by HWCs. In the following step, individuals under age 30 will be excluded [3] to form the screening pool. The screening tool will be defined as the individual eligible for screening (> 30 years of age). It is essential to consider how many persons participate from those intended or targeted and their characteristics (Supplementary Table 1). The mean age and gender distribution (proportion) for the excluded pool will also be recorded.
Diabetic pool: Those eligible for screening and invited for screening in two arms. Reasons for refusals will be noted, and in-depth interviews for in-depth exploration will be planned with participants.
The participants will be recruited into two arms to undergo non-ophthalmologist-based DR screening at the HWC in arm I and community settings in arm II, retinal image grading by trained human graders. A 2 × 2 matrix table will calculate the diagnostic accuracy measures, such as sensitivity and specificity for the HG and AI algorithms in the two study arms. The differential rate of participation or attrition (%) will be measured in terms of PwDM characteristics, or the reason for the dropout will be explored through telephone interviews through a predefined questionnaire (Supplementary Table 1).
The issue of participation in an intervention is related to the health system and participant-level factors that determine the adoption of an intervention. The purpose is to assess the adoption of intervention by the PwDM in two arms and the staff involved in implementation (Supplementary Table 1). It also involves understanding barriers to adoption and how the intervention fits organizational priorities and existing workflow from the healthcare providers (HCPs) and Program Officers (PO) perspectives.
The implementation within the framework refers to the extent to which intervention agents adhere to an intervention’s different components, including the intervention’s delivery of intervention and time (Supplementary Table 1).
Cost and time: The cost-effectiveness of the intervention will be described in objective two, and the time involved in delivering the intervention will be recorded through the time flow sheet.
Outcome (s)
Expected outcomes: This screening program was intended to understand the operational challenges in implementing two DR screening approaches. The options considered will generate evidence to design a systematic DR screening program that can increase the cost-effectiveness, scalability, reproducibility, and sustainability for integration into the public health system.
Primary outcome: clinical effectiveness (active screening)
- Proportion of people identified with DR and referred via each screening category
- Proportion of people’s adherence to the follow-up instructions after the screening
The maintenance will not be recorded in this study.
Study design
A pragmatic three-arm observational study will be conducted in primary healthcare facilities in Punjab, India. This mixed methods approach will evaluate the feasibility and cost-effectiveness of implementing different DR screening models in primary care settings. It will also generate evidence from PwDM and HCPs on barriers to accessing DR screening in public health facilities.
Study setting
The study will compare three equal-sized groups of PwDM to be recruited at primary healthcare facilities [28]. The primary healthcare settings include community settings and HWCs. Arm I will recruit PwDM at the HWC, and arms II and III will recruit PwDM in the community settings (home). The process of screening will vary by individual study arm:
Arm I: Non-ophthalmologist fundus image capture (HWC) and human grading
Arm II: Non-ophthalmologist fundus image capture (community) and AI grading
Arm III: Standard care, counseling, and referral
Study participants
People with type 2 diabetes mellitus (T2DM) aged 30 years and more will be invited for DR screening in arms I, II, and III.
Sample size
The sample size of the study was calculated based on a binary outcome (DR – yes/no) and equal sample sizes in all groups, assuming alpha = 0.05 (1.96) and power = 0.80 (beta = 0.20) [29].
n = [(a+b)2 (p1 q1 + p2 q2)]/x2
The detection of DR by human grading in a study conducted at Kapurthala (Punjab) was 0.16 (p1) and (q1) 0.84 [30]. Based on recently published findings, DR detection by AI was 0.28 (p2) and (q2) 0.75 [31]. We used these data to arrive at a sample size of 525. This sample was inflated by 15% to allow for loss to follow-up. Thus, the final sample size is 600 PwDM (200 in each arm).
Inclusion criteria
- People diagnosed with T2DM, aged 30 years and more, can provide consent and follow study instructions.
Exclusion criteria
- People with no history of T2DM, age less than 30 years, unwilling to provide informed consent
- History of intraocular surgery (other than cataract), injections, or laser for other retinal diseases
- Conjunctivitis, red eye, injury, or any eye inflammation
Preparatory phase
- Study data collection tool
Data capturing tools for quantitative data, in-depth interview guides for qualitative data, and a standardized comprehensive template for cost-effectiveness data will be developed (Supplementary Table 2).
Quantitative questionnaire: Patient-specific data include sociodemographic (age, gender, employment, education), medical history (duration of diabetes, diabetes medication history, family history of diabetes), comprehensive eye examination, and fasting blood sugar (FBS) results. Anthropometric measurements such as height, weight, waist circumference (WC), and neck circumference (NC) will be recorded (Supplementary Table 2).
In-depth interview guide (qualitative): An interview guide will be developed to explore critical stakeholder perspectives and experiences in participant background information and individual and health system barriers to access DR screening (Supplementary Table 2).
Prior permission will be obtained from the state and district health authorities to conduct the study.
Written informed consent will be obtained from the consenting individuals, and the study has obtained its approval from the Institutional Ethics Committee (IEC) of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh India, IEC approval no-PGI/IEC/2020/000741. The study will be conducted following the Declaration of Helsinki. The study protocol is registered with the Clinical Trials Registry-India (CTRI). The registration number is CTRI/2022/10/046283.
- Study implementation phase
The following activities will be undertaken before the implementation of the final study (Figure 1):
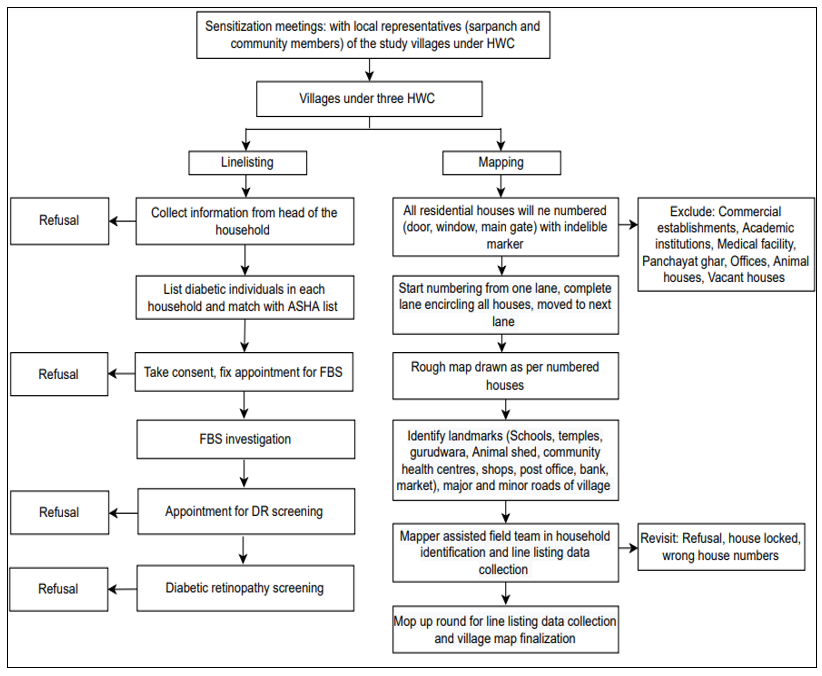 Figure 1: The following activities will be undertaken before the implementation of the final study.
Figure 1: The following activities will be undertaken before the implementation of the final study.
Stakeholder sensitization meetings: Sensitization workshops will be conducted with Medical Officer (MO), community leaders (sarpanch), accredited social health activists (ASHAs), and Community Health Officers (CHOs) to sensitize stakeholders about the activities to be conducted before, during, and after the study. Sensitization workshops will also be organized for MO, community leaders (sarpanch), ASHAs, and CHOs.
Line listing and mapping of the village households: Line listing is a list of households that details the information of the head of the household and the medical history (history of diabetes, hypertension, ocular history) of the family members. A household is a group of people who usually live together and take their meals from the same kitchen (same cooking pot) [32].
Line listing activity includes enumerating all the households in the villages within the selected HWCs. A dedicated team of field investigators, mappers, and field supervisors will collect line listing data on a predesigned form.
Mapping will involve creating a map of the area (village) to facilitate the identification of eligible households during further visits (Figure 1). The mapper will follow the field investigator and mark the unique household number on a prominent house surface (door, window, main gate) with an indelible marker. The mapper will create a map to outline the village, showing individually numbered houses along with prominent landmarks such as animal sheds, boundaries with neighboring villages, minor and major roads, schools, and religious places to serve as landmarks to identify the households during further visits (Figure 1).
Household visit for diabetes status confirmation: The mapper will guide the field team to arrive at the households with at least one member who meets the inclusion criteria. Telephone confirmation will be taken a day before visiting the households for FBS testing. The field team will arrive at the targeted family for FBS before people leave for work and have eaten, and an appointment for the next visit will be fixed. Biomedical waste generated while performing FBS will be disposed of per the guidelines [33]. The study will cover participants who decline FBS but consent to undergo DR screening.
The confirmation of the diabetic status of the patients will be performed by matching one or more of the following criteria:
- Diabetes is self-reported, or there are medical records, a prior clinical diagnosis, or medicine sachets.
- FBS testing (≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 h) [1]
- HbA1c values (≥6.5% (48 mmol/mol) (within six months) [1]
Invitation for DR screening: An appointment card with the screening date will be given to all the individuals who agree to DR screening. They will be asked to carry the card to the screening center (HWC). A reminder telephone call will be made a day before the prescribed screening date to confirm the participant’s attendance. The enrollment of the study participants will be carried out first in arm I, arm II, and arm III consecutively (Figure 2).
Data collection and analysis
Objective 1: comparing DR screening interventions (pragmatic trial)
- Anthropometric measurements: Height, weight, WC, and NC will be measured using digital scales, portable stadiometer, and stretchable tape (Figure 2).
- Blood pressure (BP): Measurements will be recorded in a sitting position, in the suitable arm, to the nearest two mmHg using the mercury sphygmomanometer. Study values will mean two BP readings taken five minutes apart.
- Visual acuity (VA) measurement: Multi-letter Snellen or E chart will record VA (Figure 2).
Nonmydriatic fundus imaging: A trained non-ophthalmologist will capture two fields (macula and disc) of non-stereoscopic per-eye images at a 45-degree field of vision (FOV). The fundus imaging in arm I participants will be carried out through a tabletop fundus camera, and a handheld camera will be used in arm II participants (Figure 2).
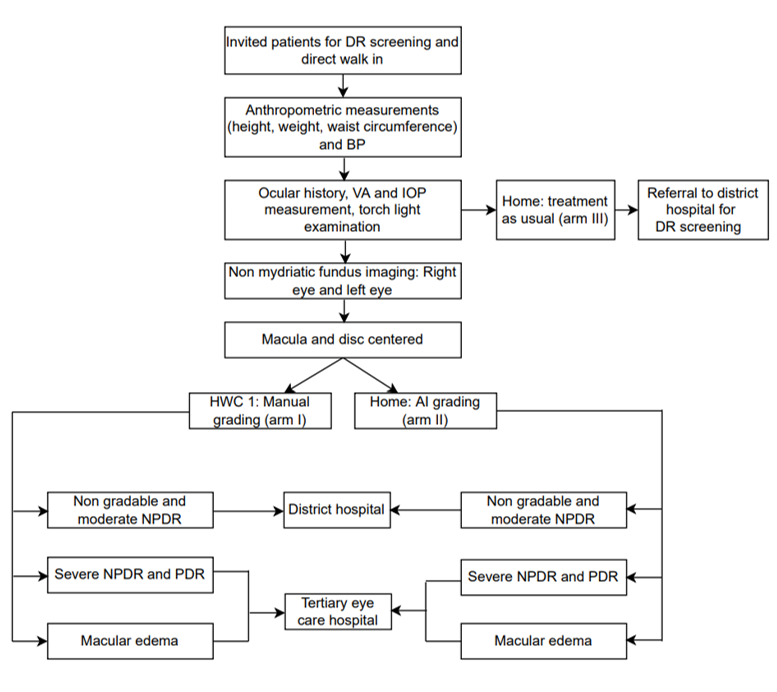 Figure 2: Diabetic retinopathy screening activities.
Figure 2: Diabetic retinopathy screening activities.
- Retina imaging modalities
Benchtop fundus camera for arm I: The benchtop camera is supported by a laptop that works on MS Windows 10 and above with a 64-bit operating system and an i3 10th generation processing system.
Smartphone-based fundus camera for arm II: This camera can be used as a handheld device or with its mounted stand. This camera is lightweight and portable, with a power backup for screening in community settings.
- Diabetic retinopathy grading
Initial grading: point of care
The initial grading (DR-yes/no) of the fundus images on the screening day will be performed by a non-ophthalmologist in arm I and an offline AI algorithm in arm II.
Final grading
The fundus images from the tabletop and handheld cameras will be uploaded to the cloud with access to the grading personnel only.
The International Council of Ophthalmology (ICO) guidelines for DR grading will be used to assess all fundus photographs [6]. Images will be analyzed for the following attributes: a) image gradability: “gradable” or “non-gradable,” b) presence of DR: “yes” and “no,” c) grading of DR: “mild non-proliferative diabetic retinopathy (NPDR),” “moderate NPDR,” “severe NPDR,” and “PDR,” and d) referral status: “yes” or “no”.
A certified optometrist and a retina-trained ophthalmologist will grade all the images separately, and a senior retina specialist will resolve any disagreements on the DR grading results. The patient will be requested to wait a week until the fundus images are finally graded by a retina-trained ophthalmologist (mild NPDR, moderate NPDR, severe NPDR, and PDR). The final reports will be shared with the patients over the WhatsApp platform or through the network of ASHA workers within eight days of the screening [34].
It is recommended that no DR and mild DR patients be counseled to visit a DH (secondary care) for a review within one year. The cases with moderate NPDR and ungradable images will be referred to the DH for further review by an ophthalmologist [35]. Severe NPDR, PDR, and macular edema will be referred to a tertiary eye institute for further evaluation and management within two weeks [34].
The diabetic patients in arm III will undergo anthropometric measurement, VA measurement, and nonmydriatic fundus imaging. The patients will be counseled on diabetes risk factors, treatment, and long-term complications. All participants in arm III will be advised to visit the DH, irrespective of DR (yes/no), for further review by an ophthalmologist. Disclosure of fundus image results will be limited to those diagnosed with severe NPDR and PDR. These individuals will be advised to seek care at a tertiary care institute. All individuals included in arm III will be contacted telephonically one month later to gather information regarding their compliance with the referral recommendations. The reasons for not visiting and the diagnosis of those who visited will be recorded (Figure 3).
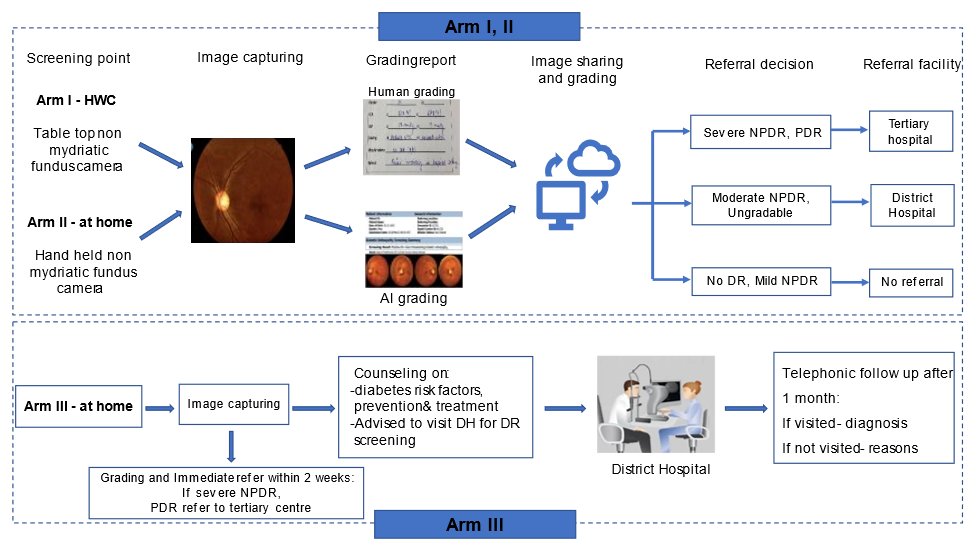 Figure 3: Screening pathway in study arms.
Figure 3: Screening pathway in study arms.
All the participant data will be entered into REDCap [36], exported in XLS format, cleaned, and then imported into and analyzed using Stata Version 15 SE [37]. A unique identification number will anonymize the identity of each participant. REDCap is browser-based, metadata-driven EDC software for designing research databases [36]. It is a secure web-based application for building and managing online surveys and databases, and the data are encrypted and password-protected through the https protocol [36]. A secure login and password will be used for data extraction.
Appropriate quantitative analyses will include descriptive statistics. Histograms will be viewed to assess the normality of continuous variables. Summary statistics will be presented as frequencies (percentages) for categorical variables and means (standard deviations) for continuous variables that are usually distributed; otherwise, medians and interquartile ranges will be reported. The level of agreement between the human graders and AI will be analyzed by calculating Cohen’s kappa coefficient statistic. The AI platform’s sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for DR detection (yes/no) will be computed using 2 × 2 tables. A P value < 0·05 will be considered statistically significant.
Objective 2: barriers to DR screening
The stakeholder groups will be organized within two main categories, focusing on using evidence to inform health practice. The providers will include retina specialists, ophthalmologists, optometrists, MO, ASHA collectively called HCPs, and the T2DM participants (Table 1).
| Category | Key informant | Examples | Number of interviews |
| Health care providers | Clinicians | Healthcare professionals (ophthalmologist, medical officer) | 2–4 |
| Community health workers | ASHA | 2–4 |
| CHO | 2–4 |
| Optometrist | 2–4 |
| Patients | PwDM | | 12–15 |
Table 1: Key informants for the in-depth interviews. ASHA: Accredited Social Health Activist; CHO: Community Health Officer; PwDM: Patient with diabetes mellitus.
- Conducting in-depth interviews
The interview guide will be designed to probe critical stakeholders’ perspectives on DR screening and identify barriers to accessing DR care. Investigating the perspectives of different key stakeholders will ensure that the heterogeneity in views and attitudes is explored. The in-depth interviews will facilitate an understanding of multiple barriers to access to DR care and allow the formulation of approaches to address the gaps. Written consent will be obtained from these key informants. The interviews with PwDM and ASHA will be conducted by a trained qualitative researcher in the local language (Punjabi) and Hindi and in English with the other stakeholders. A researcher will record, transcribe, and translate all the interviews into English. The interviews will be conducted until data saturation is achieved [38, 39]. Data saturation can be explained when no additional data can be used to develop properties of the category [40]. The data sources used are shown in the table (Table 2).
| Tool name | Place of data collection | Sequence |
| History recording sheet | HWC, at home | On the day of the screening |
| The diabetic patient interview guide | HWC, at home | After screening |
| Healthcare provider interview guide | Concerned offices, clinics | After screening |
| Cost-effectiveness tool | HWC, district hospital, tertiary eye care hospital, community settings | On the day of screening, after screening |
Table 2: Data collection tool place and sequence. HWC: health and wellness centre.
The analysis approach will involve 1) transcription, translation, and checking data accuracy with recordings, 2) coding from interview responses, 3) analyzing the remaining responses to include new codes, 4) identifying themes and subthemes from the final code structure, 5) categorizing codes under themes and subthemes, and 6) interpretation and reporting.
Objective 3: Cost-effectiveness of delivering DR screening interventions
Data for cost-effectiveness analysis will be collected using 1) the standardized comprehensive described above. This form will collect information on direct and indirect healthcare costs involved in DR screening from a health system perspective. The direct cost will include the cost of infrastructure, screening equipment, consultation, and investigations, and these data will be used to estimate the cost of implementing the two proposed DR screening programs (arms I and II).
Our study will estimate the costs of staff, consumables, and reusable equipment associated with each stage of the diagnostic pathway. It will also evaluate the proportion of indirect costs (e.g., power, light, building space) associated with each step of the diagnostic pathway. All the involved costs will be reported in 2021 Indian National Rupees (INR) with cost inflated to this year if necessary, using the Reserve Bank of India (RBI) inflation indices [41].
The costs will be combined with the data on the diagnostic performance to estimate the incremental cost per additional true positive of DR detected for the comparison of human grader diagnosis with AI diagnosis. This will be done using a decision tree model, including deterministic and probabilistic sensitivity analysis. Deterministic sensitivity analysis will be used to explore the impact of alternative assumptions around the derivation of costs. Probabilistic sensitivity analysis will be performed by assigning parameters in the model a statistical distribution and sampling from these distributions to provide distributions around model outputs (costs, diagnostic performance, and cost-effectiveness).
![]() *1, Chauhan A*1, Gupta V
*1, Chauhan A*1, Gupta V![]() 1, Kankaria A
1, Kankaria A![]() 2, Sood N3, Miglani V
2, Sood N3, Miglani V![]() 1, Tigari B
1, Tigari B![]() 1, Kaur G1, Kumar L1, Naveen M1, Budhija D1, Badada SK1 and Vale L4
1, Kaur G1, Kumar L1, Naveen M1, Budhija D1, Badada SK1 and Vale L4


