Coronaviruses are a large family of different viruses, that were discovered and characterized by British virologist in 1965. They were named after their characteristic spikes, which have a crown like appearance [1]. They are responsible for upper respiratory infections, causing common cold like symptoms. In 2002–2003, a coronavirus from southern China, spread throughout the world with a lightning speed. This novel severe acute respiratory virus was called, severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV). SARS infection was reported in 29 countries. All coronaviruses, develop in the cytoplasm of infected cells, budding into cytoplasmic vesicles from the endoplasmic reticulum. Respiratory virus infections are the most common and frequent infections of humans. These viruses enter through the respiratory tract, and cause disease that is restricted to the respiratory epithelium. The high rate of reinfection of mucosally restricted viruses, reflects the difficulty of maintaining a high level of immunity, at the vast surface of the mucosa [2]. Influenza virus is a single-stranded negative sense RNA genome virus. The well-known viruses of this group, known for causing pandemic deaths are: H1N1, which caused the 1918 pandemic, H2N2, which caused the 1957 pandemic of avian influenza, H3N2 which caused the pandemic in 1968. Two strains of coronavirus are known to cause disease in humans: coronavirus 229E (HCoV-229E), and HCoV-OC43. SARS-CoV, which spreads mainly through a respiratory route causes a unique form of viral pneumonia.
SARS causing coronavirus (SARS-CoV), coronavirus 2019 (COVID-19), has caused an unprecedented global pandemic. In the last hundred days, it has caused more death, than the number of deaths in the wars that the US has fought in this century. According to the World Health Organization (WHO) tracking, at the time of this writing, it has infected more than 6.5 million individuals worldwide and killed over 400,000. These viruses enter the nasal epithelial cells, using the surface spike proteins, to bind a metalloprotease enzyme called, angiotensin-converting enzyme 2 (ACE2), which serve as receptors for 2019-nCoV, on the bronchial epithelial cells and type 11 pneumocytes. Researchers have analyzed the ACE2 RNA expression profile at single cell resolution. High ACE2 expression has been identified in type 11 alveolar cells of lung, esophagus, enterocytes of ileum and colon, cholangiocytes, myocardial cells, kidney proximal tubule cells, and bladder urothelial cells. Based on these observations, the authors have concluded that these organs with high ACE2-expressing cells, should be considered as potential risk for 2019-nCoV infection [3, 4]. Earlier studies have demonstrated ACE2 expression in vascular endothelium and arterial smooth muscle cells [5]. These observations open a whole new way for exploring the pathogenesis, and clinical manifestations of coronavirus disease.
Prof. Beverly Hunt is the Medical Director of the British Thrombosis UK, as well as the chair for World Thrombosis Day, and works for the National Health Service, UK. It is very well known that the 2019 novel coronavirus can enter the lining of the blood vessels, by binding with the ACE2 receptor on endothelial cells. It seems that the new coronavirus behaves in some way like the conductor of the blood clotting orchestra, according to Prof. Hunt. “I’ve never seen such sticky blood”, says Prof. Beverly Hunt of NHS, UK. Initial reports from Wuhan, China, suggested that there were major clotting problems in COVID-19 patients. In a multicenter retrospective study done in Wuhan, China, in March of this year, researchers included all patients admitted with COVID-19 at the Jinyitan Hospital and Wuhan Pulmonary Hospital [6]. A total of 191 patients were included in the study. Contributing factors for severity and death included, systemic proinflammatory cytokine storm, plaque rupture, induction of procoagulant factors, hemodynamic changes, which predisposes to ischemia and thrombosis. In a prospective study of 41 patients admitted to a designated hospital in Wuhan, China, reported high amounts of IL1B, IFNγ, IP1O, MCPI. They also observed cytokine storm with the severity of the disease [7]. Gao and associates from China studied 133 patients form 17 provinces, who were admitted with influenza A virus. They found cardiac injury as frequent condition, among hospitalized patients with influenza A virus [8].
As more and more hospitalized COVID-19 patients were studied, it became evident that the risk of blood clots was greater for COVID-19 patients than other SARS infected severely ill patients [9–11]. “But the gravity of these clotting in COVID-19 patients, only became clear over the past three to four weeks, as we have started seeing these patients here,” says Dr. Behood Bikedeli, a cardiovascular medicine fellow at Columbia University, Irving Medical Center and a specialist in thrombosis. Bikedeli et al., gathered more than 40 experts in cardiology and thrombosis, from around the world to create consensus guidelines for treating clots in COVID-19 patients [12]. In a first of a kind report in JAMA Original Investigation series, Chinese researchers reported the results of a retrospective observational study done in three centers in Wuhan, China, showing neurological manifestation of the hospitalized patients with COVID-19. Of the 214 patients studied, 36.4% had various neurologic manifestation involved in CNS, PNS, and skeletal muscles. The authors concluded that rapid deterioration or worsening could be associated with neurologic events such as stroke, which would contribute to its high observed mortality [13].
Cassandra Willyard wrote in Nature News (May 13, 2020), – “Coronavirus blood-clot mystery intensifies.” Researchers are beginning to untangle the mysteries, as to why COVID-19 patients have purple rashes, swollen legs, pink toes, clogged catheters, hypoxia, and sudden death. Pathologists see the tell-tale signs of the SARS virus as well as a storm of blood clots, in every tissue and organ. Clinical biochemists see elevated D-dimers, proinflammatory markers, increased neutrophil counts, neutrophil extracellular nets (NETs), markers of prothrombotic state and signs of thrombolysis. Researchers have also observed, miniature clots in the body’s smallest vessels [14]. Researchers from University of Zurich demonstrated, endothelial cell involvement across vascular beds of different organs, in patients with COVID-19. Based on their observations they suggest therapies, to stabilize the endothelium while tracking viral replication, particularly with anti-inflammatory cytokine drugs, ACE inhibitors, and statins [15]. They suggest that this approach could be particularly relevant for vulnerable patients, with pre-existing endothelial dysfunction, which is associated with smoking, hypertension, diabetes, obesity, and established cardiovascular disease, all of which are associated with adverse outcomes in COVID-19 [16–19].
Dana Smith in an article in ‘Elemental’, titled, “Coronavirus may be a blood vessel disease”, tries to build support for this possibility. According to Prof. Mehra of Harvard University, the virus enters the lung, destroys the lung tissue, breaks open some blood vessels, then starts to infect endothelial cells, initiates a local immune response, and inflames the endothelium. He further elaborates, “A respiratory virus infecting blood cell, and circulating through the body, is virtually never heard of.” Depending upon their expertise, clinicians keep promoting the ideas, that they are familiar. For instance, Dr. Sanjum Sethi again from Columbia University says, “Inflammation and endothelial dysfunction is linked towards worse heart outcomes, in particular myocardial infarction or heart attack.” On the other hand, Prof. Beverly Hunt of NHS, UK, talks of patients, who have incredibly sticky blood, which may precipitate deep vein thrombosis (DVT). She suggests the use of small amounts of blood thinners to reduce the DVT risk.
The entire vascular bed is lined by a monolayer of endothelial cells. The normal hemodynamics is maintained by a fine balance between the vasoactive compounds generated between the vessel wall components and the circulating blood components. As an example, we have shown (Figure 1), a schematic representation of the role of vasoactive metabolites of arachidonic acid and L-arginine generated by the endothelial cells as the mediators of vasodilation. We also have shown that the synthesis of these endogenous vasodilators is inhibited by lipid hydroperoxides and oxidized lipoproteins formed in the circulating blood.
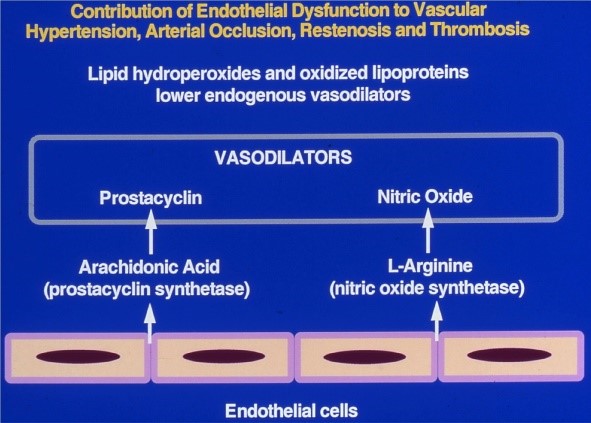
Figure 1: Contribution of endothelial dysfunction to altered hemodynamics (Courtesy: Rao GHR).
Shown in the figure, is an electron micrograph (EM) of two endothelial cells (Figure 2A). The figure represents a typical platelet interaction on denuded vascular surface devoid of endothelial cells (Figure 2B). Few red cells are trapped in the platelet-rich clot. The figure shows an EM of fibrin-rich clot (Figure 2C).
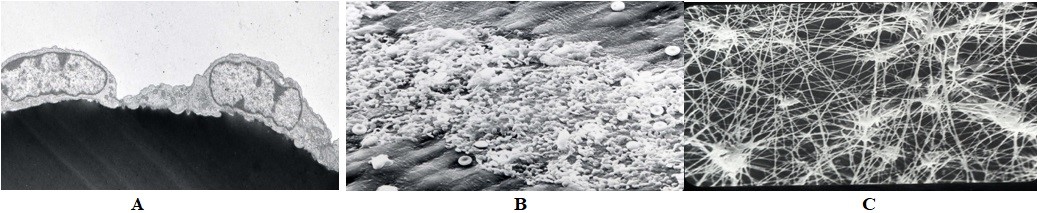
Figure 2: A: Electron micrographs of two endothelial cells, B: Platelets interacting with exposed subendothelium, C: Fibrin-rich clot (Courtesy: Late Prof. James G White).
If we start analyzing some of the recent findings, a reasonable sequalae of events occur, following the infection of SARS-CoV-2. Initial route of entry is via nasal and oral mucosa, the preferred receptor that facilitates the transmission seems to be the ubiquitous ACE2, which is found in multiple types of cells and tissue. Recent findings show that following the injury to the lung tissue, the virus gets entry into the endothelium, opens a whole new avenue for the progress of the disease and its severity [14–22]. Endothelium is the largest organ of the body, covering a large surface area and reaching out to every tissue and organ. As such, the injury to the endothelium could introduce a cascade of events, leading to platelet activation, thrombin generation, and promotion of both thrombotic and thrombolytic events. The elevation of D-dimer observed in some of the studies, indicate the occurrence of a prothrombotic event, followed by thrombolysis. In a normal situation, the thrombolytic system should clear thrombus formed, by endogenous thrombolytic agents. Researchers from University of North Carolina by infecting Serpine-1 knockout mice, have shown that the urokinase pathway had a significant effect on both lung pathology and overall SARS-CoV pathogenesis [21]. Any dysfunction of the thrombolytic pathway will create accumulation of fibrin in the vessels. This inability to cleave and clear fibrin very well may explain the very ‘sticky blood’ that Prof. Beverly Hunt talks about in her interview with the media.
Since the Zurich team’s findings were published in mid-April, dozens of studies have revelated similar patterns of vascular damage, in individuals who died of COVID-19. In a study published in the New England Journal of Medicine, researchers reported, that they found in the alveolar capillary, – thrombi 9 times as prevalent in patients with COVID-19 as in patients with influenza [20]. A multicenter collaborative study, between the Swiss Scientists and the US scientists, claims that coronavirus disease may not be a respiratory disease. They provide evidence to support, that coronavirus disease may be a vascular disease, that changes the strategies for interventions [15, 20]. Just to distinguish the term ‘vascular disease’ from the vascular damage and pathology observed in the severely ill COVID-19 patients, we refer to this condition as a ‘disease of the blood vessels’. Frank Ruschitzka, a cardiologist at University of Zurich Hospital, reported that when a patient with COVID-19 was autopsied, they found the clots and dead cells, littered the capillaries of the lungs, inflammation distended blood vessels supplying every organ in the body. Almost four decades ago, studies from our laboratory at the University of Minnesota, demonstrated the alteration in the balance of vasodilatory prostaglandin (prostacyclin) and vasoconstrictory thromboxane, synthesis in diabetic rats [23].
In majority of cases, the severity of the coronavirus disease has been found to be associated with pre-existing comorbidities, which includes metabolic diseases such as hypertension, obesity, diabetes, and vascular diseases [16–19]. Those with such diseases, will have a compromised endothelium, favoring endothelial dysfunction. The infection of endothelium by SARS-CoV-2 seems to add to this problem, by further damaging the endothelium and causing dysfunction, because of the disruption of vascular integrity and endothelial cell death. These events lead to exposure of thrombogenic basement membrane, and results in the activation of thrombotic and clotting cascade. Despite the intense unprecedented ongoing research, currently, there is no known cure for this novel disease. In view of this observation, prevention seems to be the best option, starting from hand sanitizing, wearing face masks, to social distancing, tracing the infected individuals and containment, of the COVID-19 positive individuals, as the primary modalities.
There is considerable interest in developing interventions, to prevent the interaction of spike (S) proteins of SARS-Cov-2 with ACE2 receptors. According to Center for Disease Control (CDC) report, the hospitalization rate during the 4-week period (March 2020) was 4.6% [24]. Therefore, major intervention strategies for the treatment and management of coronavirus disease, is aimed at less than 5% of the infected population. In this population, the intervention will depend primarily, on clinical manifestations diagnosed by the critical care physicians. Researchers are also working on the interventions, aimed at the prevention of lung injury, protection of endothelium from cytokine storm, and ways and means for promoting effective immune modulation. Several studies have suggested, the robust use of antithrombotic and thrombolytic therapies [16–19]. In addition, there are some limited attempts, to save the hypoxia mediated injuries and protect the tissues from destruction and ultimate death by providing oxygen therapy [18].


