Throughout life, the left ventricular (LV) goes through different remodeling patterns in response to multiple risk factors such as aging, weight, diabetes mellitus (DM), hypertension (HTN), cardiovascular diseases (CVD), and myocardial injury [1, 2]. Many other conditions are also related to left ventricular hypertrophy (LVH), including African American race, dyslipidemia, valvular stenosis, and regurgitant lesions [3–5].
Echocardiography described LV geometry patterns and the presence of LVH based on two parameters: LVM and relative wall thickness (RWT) in relation to the LV cavity. These different patterns include normal geometry (LVM and RWT are normal), concentric remodeling (CR) (increased RWT but normal LVM), concentric left ventricular hypertrophy (cLVH) (LVM and RWT are increased), and eccentric left ventricular hypertrophy (eLVH) (increased LVM with normal RWT) [6, 7].
LV geometric abnormalities are associated with impaired cardiac systolic and diastolic dysfunction. LVH can increase the risk of coronary artery disease (CAD), heart failure (HF), and arrhythmias, including sudden cardiac death (SCD), stroke, and other major cardiovascular (CV) disorders [2, 8]. LVM is an independent risk factor for the prediction of CV events. However, the best way to incorporate LVM into clinical decision-making algorithms has yet to be established [9]. Even in a normal range for healthy adults, LVM is positively related to systolic blood pressure, body mass index (BMI), and coronary calcium score by cardiac computed tomography [10, 11]. Geometrical changes could also be found in normal people; a study by Andrén et al. showed that 16% of healthy persons demonstrated the presence of LVH (mainly eLVH) [12].
In this study, we sought to examine patterns of LV geometry determined by echocardiography and their association with cardiac pathologies.
Study design and setting
This cross-sectional study examines the clinical correlations of changes in LV geometric patterns among patients at King Abdulaziz Hospital in Al-Ahsa from July 2020 to December 2022. Patients were randomly selected from the cardiology inpatient and outpatient services, with no exclusion criteria. This study included 260 patients, men and women aged 15–92 years old. The confidentiality of participants’ information was assured.
Data collection
The baseline characteristics, BMI, comorbidities, medications, and type of LVH were obtained from the database system of King Abdulaziz Hospital. Two-dimensional (2D) guided M-mode echocardiograms were performed on each patient by expert sonographers using ultrasound machines; GE Vivid E95 (Probe M5Sc-D 1.4-4.6 MHz), Siemens Acuson SC2000 (Probe 4V1C transducer 1.25-4.5 MHz), and Philips Affiniti 70 (Probe Philips X5-1 xMatrix probe 1-5 MHz). Measurements were cross-checked on the EchoPAC system by two other echocardiography sonographers. Measurements were taken at or just below the tip of the mitral valve. LV internal dimension and septal posterior wall thickness were measured at end-diastole and end-systole, according to the American Society of Echocardiography (ASE) guidelines [13].
Calculations
Left ventricular mass index (LVMI) and RWT were calculated by using the formula LVMI = LVM/BSA (considering that LVM = 0.8{1.04[([left ventricular end-diastolic diameter (LVEDD) + interventricular septal thickness at end-diastole (IVSd) + posterior wall thickness at end-diastole (PWd)]3 – LVEDD3)]} + 0.6), and RWT = 2 × PWd/LVEDD. LVMI of more than 95 g/m2 in females and more than 115 g/m2 in males, and RWT of more than 42 were considered abnormal [15]. Patients were then classified into four mutually exclusive groups based on RWT and LVMI: normal geometry, CR, eLVH, and cLVH.
Statistical analysis
All participants’ data were analyzed using Statistical Package for Social Studies (SPSS 22; IBM Corp., New York, NY, USA). Cox proportional hazards models were used to evaluate the relationships among LVMI, RWT, LV geometry, and clinical diagnosis. Cross-tabulation was done to assess the LV geometry according to the participant’s gender, BMI, and comorbidities. The differences were tested by using the Pearson chi-square. A p-value of < 0.05 was considered statistically significant with a 95% confidence interval.
Ethical consideration
Study approval was obtained by King Abdullah International Medical Research Center (KAIMRC) through Institutional Review Board (Ethics approval number IRB/2476/22). The study was conducted per the Helsinki Declaration of 1975 and KAIMRC guidelines. Written informed consent was obtained from all study participants (or their legal guardians, where applicable) before study enrolment. All participants’ information was stored confidentially and anonymously.
We analyzed 260 patients’ data to reach a consensus about LV geometric patterns change and the determinants of these changes, of which 210 were previously labeled with LVH by echocardiogram before implementing LVMI and RWT measurements. Among 260 patients, the echocardiographic patterns of LV geometry showed normal geometry prevalence (43.46%) followed by CR (30.76%), cLVH (15.76%), and eLVH (10.00%) respectively (Table 1) (Figures 1 and 2).
| Variable | Category | Data |
| Age | Mean | 64.74 years old |
| Minimum | 15 years old |
| Maximum | 92 years old |
| Gender | Male | 112 (43.07%) |
| Female | 148 (56.93%) |
| Body mass index (BMI) | Average | 30.28 kg/m2 |
| Left ventricular mass index (LVMI) | Average | 69.81 gm/m2 |
| Relative wall thickness (RWT) | Average | 0.65 |
| Type of left ventricular (LV) geometry shown in echocardiogram | Concentric hypertrophy | 41 (15.76%) |
| Concentric remodeling | 80 (30.76%) |
| Eccentric hypertrophy | 26 (10.00%) |
| Normal geometry | 113 (43.46%) |
| Coexisting non-cardiac conditions | Diabetes mellitus (DM) | 187 (71.92%) |
| Hypertension (HTN) | 222 (85.38%) |
| Dyslipidemia | 141 (54.23%) |
| Hypothyroidism | 29 (11.15%) |
| Others (ESRD, stroke, anemia, etc.) | 75 (28.84%) |
| Cardiac conditions | Atrial fibrillation (AF) | 42(24.3%) |
| Coronary artery disease (CAD) | 96 (36.9%) |
| Heart failure (HF) | 77 (29.6%) |
| Valvular disease (VD) | 26 (10%) |
| Arrhythmia | 15 (8.7%) |
Table 1: Demographic characteristics of the included patients (N = 260).
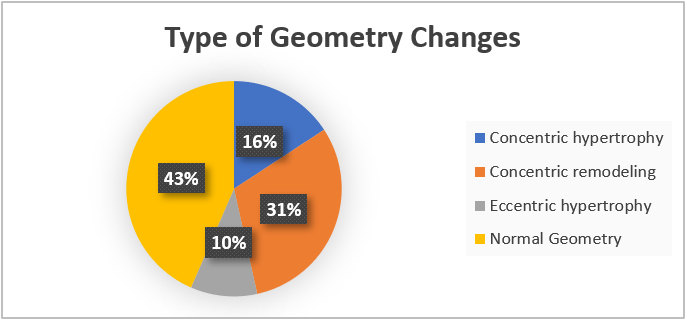 Figure 1: Types of left ventricular (LV) geometry and percentage.
Figure 1: Types of left ventricular (LV) geometry and percentage.
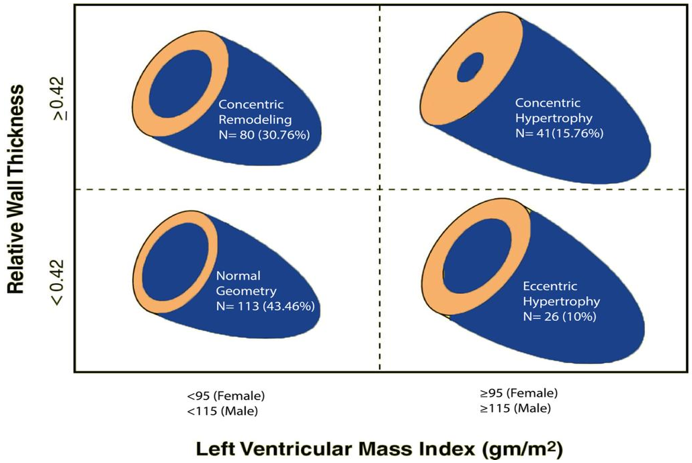 Figure 2: Relative wall thickness (RWT) and left ventricular mass index (LVMI).
Figure 2: Relative wall thickness (RWT) and left ventricular mass index (LVMI).
The mean age of the patients was 64.74 years of age, the maximum age was 92 years old, and the minimum age was 15 years old. The sample was divided into different age groups, which showed various LV geometry patterns. The most common age group was 55–56 (35%), followed by age groups 66–76 (30%), above 77 (19.2%), 44–54 (11.2%), 33–43 (1.9%), 22–32 (1.5%), and 11–21 (1.2%), respectively (Figure 3).
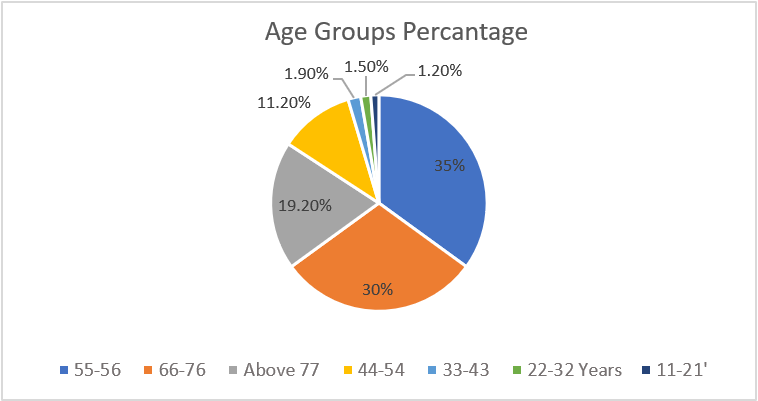 Figure 3: Pie chart of percentages of the different age groups.
Figure 3: Pie chart of percentages of the different age groups.
More than 55% of the cohort were female in gender. Moreover, we analyzed gender differences to see which type of geometry changes is more common in both genders. No significance between gender and type of LV geometry pattern was found, and normal geometry followed by CR was the commonest, while the least was eLVH. Further details about the correlation and the gender-specific analysis are found in the table (Table 2).
Further analysis of left ventricular (LV) geometry |
| Female | Male | Total |
| Type of LV geometry | Normal geometry | Number (%) | 59 (39.6%) | 54 (48.6%) | 113 (43.5%) |
| Concentric remodeling | Number (%) | 47 (31.5%) | 33 (29.7%) | 80 (30.8%) |
| Concentric hypertrophy | Number (%) | 24 (16.1%) | 17 (15.3%) | 41 (15.8%) |
| Eccentric hypertrophy | Number (%) | 19 (12.8%) | 7 (6.3%) | 26 (10.0%) |
| The correlation between gender and LV geometry changes* | p-value* | 0.269* |
Table 2: The correlation between left ventricular (LV) geometry changes and gender (N = 260). *Pearson correlation coefficient, a p-value of 0.05 was considered significant.
The average BMI was found to be 30.28 kg\m2. The echocardiogram of these patients showed an average of 69.81 gm\m2 as the LVMI and the RWT was 0.65 on average. The commonest type of LV geometry was normal geometry (> 43%), followed by CR (> 30%), while the least was eLVH (10%) (Table 3) (Figure 2).
| Type of left ventricular (LV) geometry | Total |
| Normal geometry | Concentric remodeling | Concentric hypertrophy | Eccentric hypertrophy |
| Body mass index (BMI) category | Underweight | Count | 0 | 2 | 1 | 0 | 3 |
| % Within LV geometry | 0.0% | 2.5% | 2.4% | 0.0% | 1.2% |
| Normal | Count | 24 | 8 | 9 | 3 | 44 |
| % Within LV geometry | 21.2% | 10.0% | 22.0% | 11.5% | 16.9% |
| Overweight | Count | 26 | 21 | 12 | 5 | 64 |
| % Within LV geometry | 23% | 26.2% | 29.3% | 19.2% | 24.6% |
| Class 1 obesity | Count | 45 | 39 | 13 | 12 | 109 |
| % Within LV geometry | 39.8% | 48.8% | 31.7% | 46.2% | 41.9% |
| Class 2 morbidly obese | Count | 12 | 8 | 4 | 6 | 30 |
| % Within LV geometry | 10.6% | 10.0% | 9.8% | 23.1% | 11.5% |
| Class 3 severe obesity | Count | 6 | 2 | 2 | 0 | 10 |
| % Within LV geometry | 5.3% | 2.5% | 4.9% | 0.0% | 3.8% |
| Count | 110 | 80 | 41 | 26 | 260 |
Table 3: Body mass index (BMI) category and type of left ventricular (LV) geometry pattern crosstabulation.
The commonest non-cardiac condition was found to be HTN (> 80%), followed by DM (> 70%), while the least common was found to be hypothyroidism (11.15%). Among HTN patients, the commonest LV geometry was normal geometry, followed by CR (Table 4).
As for the cardiac conditions in our population, the commonest encountered CVD was CAD, followed by other cardiac conditions (HF, atrial fibrillation (AF), valvular heart disease (VHD), and arrhythmia). Further details about the patients’ demographic characteristics are found in the table and figure (Table 1 and Figure 4).
| Hypertension (HTN) | Total |
| no HTN | with HTN |
| Type of left ventricular (LV) hypertrophy/remodeling | Normal geometry | Count | 20 | 93 | 113 |
| % Within HTN | 52.6% | 41.9% | 43.5% |
| Concentric remodeling | Count | 7 | 73 | 80 |
| % Within HTN | 18.4% | 32.9% | 30.8% |
| Concentric hypertrophy | Count | 8 | 33 | 41 |
| % Within HTN | 21.1% | 14.9% | 15.8% |
| Eccentric hypertrophy | Count | 3 | 23 | 26 |
| % Within HTN | 7.9% | 10.4% | 10.0% |
| Total | Count | 38 | 222 | 260 |
| % Within HTN | 100.0% | 100.0% | 100.0% |
Table 4: Type of left ventricular (LV) geometry and hypertension (HTN) crosstabulation.
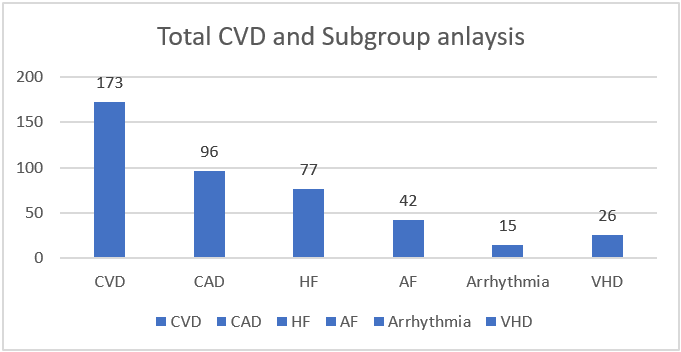 Figure 4: The prevalence of specific cardiac disorders.
Figure 4: The prevalence of specific cardiac disorders.
We analyzed the LV geometry changes to conclude its determinants and the correlated variables. The difference in gender, BMI, age, and other non-cardiac conditions, such as DM, HTN, and others, was non-significantly associated with LV geometry changes. In addition, further analysis was done to find the correlation between the presence of CVD and LV geometry changes. We identified a strong association between the presence of CVD and the LV geometry changes in the echocardiogram (p-value 0.005); however, when further sub-group analysis was done to find which cardiac disease is associated with a specific type of geometry change, the correlation was non-significant, (p-value 0.736). Moreover, no correlation was found in relation to LVMI, RWT, and specific cardiac disorders, with a p-value of 0.028 and 0.715, respectively. More details about the correlation and the CVD are found in the tables below (Tables 5, 6, and 7).
| CVD (No) | CVD (Yes) |
Total
|
| Type of LV geometry | Normal geometry | Number (%) | 44 (54.3%) | 69 (38.5%) | 113 (43.5%) |
| Concentric remodeling | Number (%) | 29 (35.8%) | 51 (28.5%) | 80 (30.8%) |
| Concentric hypertrophy | Number (%) | 8 (9.9%) | 33 (18.4%) | 41 (15.8%) |
| Eccentric hypertrophy | Number (%) | 0 (0.0%) | 26 (14.5%) | 26 (14.5%) |
| The correlation between the presence of CVD and LV geometry changes* | p-value* | 0.005* |
Table 5: The correlation between left ventricular (LV) geometry changes and the presence of cardiovascular disease (CVD) (N = 260). *Pearson correlation coefficient, a p-value of 0.05 was considered significant.
| Type of left ventricular (LV) geometry | p-value |
| Normal geometry | Concentric remodeling | Concentric hypertrophy | Eccentric hypertrophy |
| Cardiac disease | Atrial fibrillation (AF) | 9 | 21 | 12 | 6 | 0.002 |
| Coronary artery disease (CAD) | 41 | 21 | 17 | 17 | 0.029 |
| Heart failure (HF) | 30 | 14 | 13 | 20 | < 0.001 |
| Valvular heart disease (VHD) | 4 | 9 | 9 | 4 | 0.003 |
| Arrhythmia | 5 | 6 | 2 | 4 | 0.392 |
| Others | 2 | 0 | 2 | 0 | 0.177 |
Table 6: Sub-group analysis of the common cardiovascular disease (CVD) and the left ventricular (LV) geometry changes (N = 260).
| p-value** | Lower C.I.*** | Upper C.I. |
|
Groups of CV disorders*
| Correlated to LV geometry | 0.736 | ——– | ——— |
| Correlated to RWT | 0.028 | -0.178 | 0.124 |
| Correlated to LVMI | 0.715 | 33 (18.4%) | 41 (15.8%) |
Table 7: Sub-group analysis of common cardiovascular (CV) disorders and left ventricular (LV) geometry changes (N = 260). *The disorders analyzed were as follows: atrial fibrillation (AF), heart failure (HF), arrhythmia, valvular heart disease (VHD), coronary artery disease (CAD); **p-value less than 0.05 was considered significant; ***C.I. stands for the confidence interval (95%).
In response to multiple risk factors such as aging, obesity, DM, HTN, and CVD, the LV undergoes different remodeling patterns throughout a person’s life [1, 2]. LVH by ECG has been since long time considered a powerful CV risk factor [14].
Following the introduction of echocardiography in epidemiological studies, it was found that LVH prevalence was grossly underestimated using ECG detection alone. Then cardiac magnetic resonance (CMR) was added to echocardiography to assess LVM. Echocardiography may be used to evaluate LVM in 2D or 3D. M-mode was the first noninvasive imaging technique pioneered and is the recommended method [15, 16].
For the quantification of LVM, CMR provides a 3D high-resolution model of the LV that is highly accurate [17]. Despite a high correlation between CMR and echocardiography LVM measurements, absolute values of LVM may differ between both [18, 19]. Compared to CMR, echocardiography is less expensive and versatile, and LVM from echocardiography is a good predictor of future events. Therefore, the ASE-recommended formula should be reported across all echocardiogram laboratories.
Current guidelines recognize LVH as a potentially debilitating form of target-organ damage in hypertensive populations; however, the inclusion of either LVM or LVH in HTN treatment algorithms varies from one to another [20–23]. This explains why the clinical use of LVM measurements has yet to be firmly established [15, 23]. In cardiac remodeling, an increase in LVM is the essential component, which results from the balance between cardiac stressors and compensatory mechanisms that have not yet been fully understood [24, 25].
Abnormalities in LV geometry
Typically, HTN and other CV disorders can include four distinct patterns of LV geometry based on the relationship between LV cavity size and RWT, including CR, eLVH, and cLVH [26]. The ASE currently defines an increased LVMI as > 115 g/m2 in men and > 95 g/m2 in women, and RWT is calculated as 2× posterior wall thickness in diastole/LV internal diameter > 0.42 [15].
Among 260 patients in our study population, the percentage of each abnormal LV geometry was CR (30.76%), cLVH (15.76%), and eLVH (10.00%), respectively (Table 1) (Figure 2).
Gender and LV geometry
It is well established that LV dilation occurs following myocardial infarction (MI). However, natural changes in LV geometry have yet to be well studied in the general population. Over 15 years of follow-up from the Framingham Heart Study showed that LVM increased among men but decreased among women. These changes were driven by increased LVEDD in men and decreased LV wall thickness in women [27]. A study by Krumholz et al. found that the geometric pattern of LVH differed by sex. A pattern of eLVH was observed mainly in men, while women showed a pattern of cLVH [28].
In our sample, the percentage of women was more than men by 55%; our study showed no significant difference between gender and the type of LV geometry pattern (p-value 0.50). In both genders, normal geometry was the most reported finding, followed by CR, with eLVH found to be the least.
Krumholz’s study included subjects without clinically apparent CVD, antihypertensive medication, and diastolic HTN [28]. Similarly, a study by Masiha et al., which had only the elderly aged 70 showed no significant interactions between gender and the LV geometric groups, with normal geometry being the highest and eLVH the least [29]. Evidence indicates that postmenopausal women’s hearts are more sensitive to trophic stimuli. cLVH was a common finding in women, and these findings were reported by Kuch et al. [30].
Body mass index and LV geometry
Obesity is one of the strongest predictors for abnormal LV geometry, including LVH, and typically obesity increases with age and is a vital risk factor for HTN [8, 31, 32]. A significant correlation exists between obesity and LVM. Increasing adiposity increases LV wall thickness and internal dimensions, leading to an increase in LVM [33]. Abnormal LV geometry, including CR and both eLVH and cLVH, are more common in obese than leaner patients, with the same impact of obesity on abnormal LV geometry noted in both women and men [32, 34].
The average BMI in our study was found to be 30.28 kg\m2. Obesity class 1 is defined as BMI (30–<35), class 2 as morbidly obese BMI (35–<40,) and class 3 as severe obesity BMI (>40), respectively. Our study’s first distinctive finding was the presence of a significant correlation with abnormal LV geometry in obesity class 1. CR was the commonest among class 1 obesity (48.8%), followed by eLVH (46.2%), normal geometry (39.8%), and cLVH (31.7%), respectively (Table 3).
Studies have demonstrated that combined HTN and obesity significantly adversely impact LV diastolic filling [8, 31]. There is a strong “obesity paradox” in many cohorts with CVD [35–37]. Despite the increased prevalence of abnormal LV geometry in obesity, better survival among obese is demonstrated compared to their leaner partners [32, 34]. In fact, for every LV geometric profile (normal LV geometry, CR, eLVH, and cLVH), obese had lower mortality than leaner patients [34].
Cardiovascular disease and LV geometry
More than four decades ago, data from the Framingham Heart Study demonstrated that definite ECG evidence of LVH, both by voltage and with repolarization abnormalities or “strain” pattern on the ECG, was associated with a 6- 8-fold increase in CAD and CV mortality, respectively [14].
Our study identified a strong association between CVD and abnormal LV geometry changes on echocardiogram, with a (p-value of 0.005). The prevalence of CVD in our population with normal LV geometry was 38.5%, while CVD in relation to abnormal LV geometry was 28.5% in CR, 8.4% in cLVH, and 14.5% in eLVH (Table 5).
The CV disorders analyzed were CAD, HF, AF, arrhythmia, and VHD. The association of LVH with a very high prevalence of CV events seems to be a more potent risk factor than conventional CV risk factors for predicting significant CV morbidity and mortality [7, 8, 38–40].
Further sub-group analysis to identify the correlation of CVD with a specific type of LV geometry change was non-significant (p-value 0.736). Moreover, no correlation was found in relation to LVMI, RWT, and specific CVD (p-values of 0.028 and 0.715, respectively). More details about the correlation and CVD are found in Tables 5, 6, and 7.
Of the 260 subjects, CVD patients were 173 (66.5%). Further subgroup analysis in relation to the CVD groups and LV geometry: 96 CAD patients (55.5%) (p-value of 0.029), HF 77 patients (29.6%) (p-value < 0.001), AF 42 patients (24.3%) (p-value 0.002), arrhythmia 15 patients (8.7%) (p-value 0.392), and VHD 26 patients (15.01%) (p-value 0.003), respectively.
The second distinctive finding in our study is the correlation of CAD in relation to abnormal LV geometry, which was more associated with eLVH. CAD was the most prevalent among our CVD population (p-value 0.029). We have yet to come across studies concluding this direct relation in the absence of systolic HF. A total of 52 subjects out of the 96 CAD subjects did not have clinical HF, indicating milder forms of CAD and may exhibit altered geometry earlier in the disease process.
HF in the CVD subanalysis demonstrated the highest significant statistical inference in relation to abnormal LV geometry (p-value < 0.001) and was more with eLVH.
Out of the 77 HF subjects, HF with reduced ejection fraction (HFrEF) was found in 60 patients (23.1%), and 37 only with HFrEF had advanced high-risk CAD. In contrast, HF with preserved ejection fraction (HFpEF) was found in 17 patients (6.5%) in relation to LV geometry.
LVH is more strongly associated with diastolic than systolic dysfunction [8, 31, 41]. Milani et al. demonstrated that 13% of patients with preserved LV systolic function and cLVH progressed to LV systolic dysfunction during a 3-year follow-up, indicating that LVH impacts diastolic and systolic HF [42].
LVH is a vital risk factor for AF [43, 44], while regression of LVH is associated with a reduced risk of AF [45]. The AF population in our study was not lone AF (total 42) (p-value 0.002) but was in conjunction with other pre-existing CVD ailments of HTN, CAD, VHD, and HF, which may explain the trend toward the combination of CR and cLVH in these subjects.
Messerli et al. demonstrated decades ago that LVH was associated with the high prevalence of arrhythmia [46]; our sample of arrhythmia subjects was tiny, with 15 patients only (p-value 0.392).
LVH and SCD are closely associated; several echocardiographic studies have demonstrated an increased risk of arrhythmia and risk of SCD, whereas regression of LVH may be associated with a reduced risk of SCD [39, 47, 48].
Hypertension and LV geometry
The LV is generally thought to adapt to sustained HTN by developing cLVH. According to the paradigm of compensatory ventricular response to chronic pressure overload, ventricular wall thickness should increase proportionally to blood level to maintain normal wall stress.
Many patients with mild to moderate HTN exhibit normal LVM and wall thickness, while others have eLVH unrelated to systolic dysfunction but rather to increased cardiac output and preload. Conversely, HTN patients can have CR, where LV dilation represents a late transition toward myocardial failure [26]. Despite different variables that did not infer statistical significance in relation to abnormal LV geometry, the commonest non-cardiac encountered determinate in our population was HTN, with a prevalence of 85%.
Through the years, several studies demonstrated the alteration of LV because of HTN. The first study showed the relationship between HTN and different patterns of LV geometry, which Ganau et al. conducted in 1992 [26]. This study included 165 hypertensive and 125 middle-aged adults and used the combination of LVM and the relation of muscle wall thickness to cavity size to describe LV geometry patterns. Among hypertensive patients, 52% had utterly normal LVM and RWT,13% had a normal LVM but increased RWT (CR), of the remaining persons, 27% had an increased mass with normal RWT (eLVH), and 8% had an increase in both variables leading to cLVH.
Another study by Krumholz et al. showed that HTN has also been associated with cLVH [28].
While cLVH and eLVH were the most frequent geometric patterns, diastolic dysfunction is present in most patients [49], with cLVH carrying the worst prognosis, followed by eLVH, CR, and normal geometry. Subjects with cLVH also had the highest LVM [7].
In contrast to these studies, our study enrolled 222 hypertensive patients; 41.9% had normal geometry, followed by CR in 32.9%; while 14.9% had cLVH, only 10.4% had eLVH. These results lean toward milder treated forms of HTN in our population.
The third distinctive finding in our study is that most subjects (210 out of 260) had preliminary echocardiogram reports of LVH before implementing LVMI and RWT measurements. Later only 113 patients (43.46%) were reported as having normal LVM and RWT. These patients were misled to believe they had LVH, while proper LVH assessment by ASE guidelines was not implemented.
An analysis from the Framingham Heart Study using LV geometric groups found that most individuals with an initially normal LV geometry retained the normal LV geometry after four years. Many individuals with an abnormal LV pattern were normalized after four years, and a few individuals with a cLVH pattern had turned into eLVH [50]. The CV health study, including elderly individuals, showed similar findings and changes in LV geometric groups over seven years [51].
The estimation of LVM by echocardiography offers prognostic information beyond that provided by evaluating traditional CV risk factors. An increase in LVM predicts a higher incidence of clinical events, including death, attributable to CVD [38].
Other correlations with LV geometry
We also explored other non-cardiac determinants of abnormal LV geometry, including pulmonary diseases (bronchial asthma, chronic obstructive airway disease, bronchiectasis, and pulmonary hypertension), DM, smoking, dyslipidemia, stroke, and kidney disease.
We found DM more prevalent (72%) after HTN (85%); the highest association with DM was with normal LV geometry (40.4%), followed by CR (63%). Dyslipidemia (53%) came third, associated with normal LV geometry (41.7%), then CR (31.7%). Obesity (41%) came fourth with CR (48.8%). All of which did not infer significant statistical correlation.
![]() *1,5, Alshaikh Husain M
*1,5, Alshaikh Husain M![]() 2, Gado W
2, Gado W![]() 1, Alghasham KZ
1, Alghasham KZ![]() 1, Faqeehi R3, Al-Raimi S
1, Faqeehi R3, Al-Raimi S![]() 3, AlMukhaylid S
3, AlMukhaylid S![]() 4, Alsultan NF
4, Alsultan NF![]() 1, Alharbi FS
1, Alharbi FS![]() 1, Altuwaim IN1, Alibrahim KI1, Aleid NF
1, Altuwaim IN1, Alibrahim KI1, Aleid NF![]() 1 and Soliman AF
1 and Soliman AF![]() 1,5
1,5



