Abstract
Introduction: The pandemic caused by the new SARS-CoV-2 virus has reported an increase in morbidity and mortality worldwide during the years 2020 to 2022. Many pediatric infections have been reported as mild. The cardiovascular complications due to COVID-19 described in the literature are mainly in adults, however, reports in the pediatric population have been less frequent.
Objective: To describe the clinical characteristics and evolution of patients treated in two centers with cardio-pediatric care services who presented cardiovascular involvement during the COVID-19 pandemic related to the presence of SARS-CoV-2 infection and Kawasaki disease (KD).
Materials and Methods: Through a descriptive, retrospective study, the medical records, electrocardiographic and echocardiographic studies were reviewed in both groups of patients between May 2020 and May 2022 at the National Institute of Child Health and Alberto Sabogal Hospital. None of the cases had a history of previous structural heart disease.
Results: The patients studied were 31 in total, with 21 cases of COVID-19 (SARS-CoV-2 infection) and 10 of KD. The female sex predominated, with an average age of 6.2 years (COVID-19) and 2.9 years (Kawasaki). In the echocardiogram, mild pericardial effusion was the most common finding. Coronary alterations were found in 60% of patients with KD and in only 18% of COVID-19 cases. We found 15 patients who met the criteria for the so-called multisystem syndrome (MIS-C) among COVID-19 cases, 5 of them with hemodynamic compromise.
Conclusion: During the COVID-19 pandemic, the clinical picture in both groups: SARS-CoV-2 infection and KD presented some similar characteristics, mainly in relation to coronary involvement (greater involvement in KD), and in the evolution, a greater hemodynamic compromise was evidenced in cases of SARS-CoV-2 infection but without associated mortality.
Keywords
coronavirus infections, Kawasaki disease, pediatrics, cardiovascular diseases
Introduction
Since the first case of COVID-19 was reported in our country in March 2020 in the adult population [1], the trend has been growing as in most countries in Latin America and the world. After 3 weeks, the first cases were registered in children [2] and subsequent hospitalizations occurred in the pediatric population due to COVID-19, a slight increase in admissions for Kawasaki disease (KD) was also observed. In the pediatric group, the so-called multisystem syndrome related to SARS-CoV-2 with the acronym MIS-C [3] has also emerged, which can also be manifested with some symptoms similar to KD, and evidence of cardiovascular compromise.
COVID-19 seems less aggressive in pediatric patients and the symptoms are milder than in adults. The most common symptoms in children are mild fever, cough, runny nose, and sore throat. In addition, gastrointestinal symptoms such as vomiting and diarrhea are also common [4, 5]. In a report from China, cardiac involvement was described in 12% of adult patients while in another study from the same country, arrhythmias and myocardial injury were reported in 16.7% and 7.2% of them [6].
Studies suggest a milder clinical course in children and a less frequent need for intensive care management [7]. There is currently limited information regarding cardiovascular and myocardial involvement in pediatric patients with COVID-19, although reports of isolated cases present with similar characteristics to KD with myocarditis are being published [8, 9].
In this regard, groups of children and adolescents have been described in Europe and North America who have had to be admitted to intensive care units suffering from a multisystem inflammatory condition with characteristics similar to KD and toxic shock syndrome. Acute clinical pictures accompanied by a hyperinflammatory syndrome progressing to multiple organ failure and shock have been described in case reports and small registered series. Based on the results of initial laboratory tests, the hypotheses suggest that this syndrome may be related to COVID-19 [10, 11].
The objective of this study is to describe the clinical evolution in both groups of patients, those with COVID-19 (infection by SARS-CoV-2) and those with KD, as they have certain similar clinical characteristics as well as in both cases the possibility of cardiovascular compromise, and also some studies mention an increase in the incidence of the latter in association with epidemic viral outbreaks being different clinical entities [12].
Materials and Methods
The clinical records of hospitalized patients diagnosed with COVID-19 (SARS-Cov-2 infection) and KD in two health institutions from May 2020 to May 2022 were reviewed.
Suspected or confirmed cases of cardiovascular compromise determined by the clinical picture (fatigue during lactation, dyspnea, palpitations, tachypnea, subcostal indrawing, profuse sweating, hepatomegaly, edema of the lower limbs, cardiomegaly, prominent left ventricular impulse) were included in the study, for which an ECG and/or Doppler echocardiography were performed. The clinical evaluation of the patients and the examinations were carried out by the pediatric cardiologists of the health institutions that participated in the research.
In addition, a database was created in which clinical signs, laboratory findings, and imaging (radiology, echocardiogram, or angiotomography) were recorded based on the WHO case notification form “Global COVID-19 clinical platform: case report form for suspected cases of multisystem inflammatory syndrome (MIS) in children and adolescents temporally related to COVID-19”.
The COVID-19 and MIS-C case definition criteria were made according to the WHO report and the Kawasaki cases satisfying the AHA-2017 criteria.
COVID-19-WHO criteria. Clinical criteria: Sudden onset of fever and cough; or sudden onset of three or more signs or symptoms from the following list – fever, cough, general weakness/fatigue, headache, myalgia, sore throat, runny nose, dyspnea, anorexia/nausea/vomiting, diarrhea, altered mental status. A person with laboratory-confirmed COVID-19 virus infection, regardless of clinical signs and symptoms.
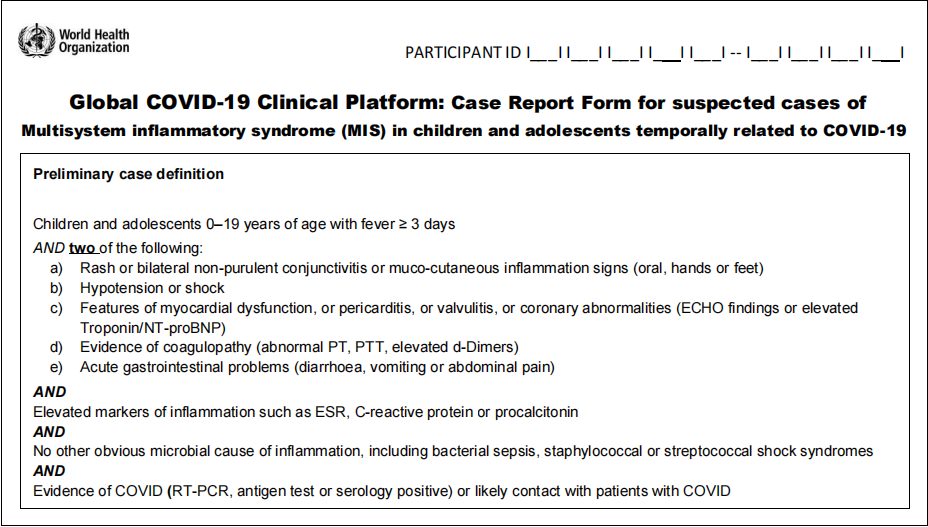
Case definition: multisystem inflammatory syndrome (MIS-C). Children and adolescents aged 0–19 years with fever for three days or more and two of the following criteria: a) skin rash or bilateral non-purulent conjunctivitis or signs of mucocutaneous inflammation (mouth, hands, or feet); b) hypotension or shock; c) features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including echocardiographic signs or elevated troponin/NT-proBNP values); d) evidence of coagulopathy (based on PT, aPTT, or elevated D-dimer levels); e) acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain). High values of inflammation markers (ESR, C-reactive protein, or procalcitonin). No other obvious microbial cause of inflammation, including bacterial sepsis and staphylococcal or streptococcal toxic shock syndromes. COVID-19 tests (RT-PCR, antigen tests, or positive serology) or possible contact with a COVID-19 patient.
 KD criteria is representative of the AHA. Classic KD is diagnosed when the patient has a fever for more than 5 days and at least 4 of the following clinical characteristics – bilateral conjunctival injection, changes in the lips and oral cavity, cervical adenopathy, changes in the extremities, and polymorphous rash. If the patient has few clinical findings but abnormalities in the coronary arteries are found on the echocardiogram, then the diagnosis can be made, since the clinical features tend to appear sequentially.
KD criteria is representative of the AHA. Classic KD is diagnosed when the patient has a fever for more than 5 days and at least 4 of the following clinical characteristics – bilateral conjunctival injection, changes in the lips and oral cavity, cervical adenopathy, changes in the extremities, and polymorphous rash. If the patient has few clinical findings but abnormalities in the coronary arteries are found on the echocardiogram, then the diagnosis can be made, since the clinical features tend to appear sequentially.
Alternative diagnostic criteria for KD include fever for at least 5 days and two or three main features, such as coronary abnormalities on echocardiography.
Echocardiographic studies were performed in the Echocardiography Cabinets of the Cardiology Services of each health institution with General Electic Healthcare and Phillips equipment, respectively, maintaining the safety recommendations for procedures in patients with suspected or confirmed COVID-19 cases (American Society of Echocardiography Procedural Guide).
For the statistical analysis, the Statistical Package for Social Sciences (SPSS) 23.0 for Windows system was used and it was based on descriptive statistics techniques. Absolute frequency distributions were made and percentages were used as summary measures for qualitative variables, mean (X), and standard deviation (SD) for quantitative variables.
Results
Clinical and demographic data are found in the table (Table 1). The predominance of female sex, girls/boys ratio: 18/13.
| COVID-19 (infection SARS-CoV-2) | Kawasaki disease |
| Number of patients | 21 | 10 |
| Age (years) | 6.2 (2.8-12.2) | 2.9 (0.8-4.8) |
| Weight (kg) | 16.5 (11.6-43.5) | 12.7 (7.2-16.6) |
| Echocardiographic findings | Mean (range) | Mean (range) |
| FEVI | 51% (39-67) | 63% (50-65) |
| DDVI (Z score) | +1.1 (+0.9, +1.6) | +0.9 (+0.7, +1.1) |
| DDVI (mm) | 26 (23-35) | 22 (18-24) |
| Mitral regurgitation (Doppler) | 7 cases (33%) | 0 cases |
| Pericardial effusion | 8 cases (38%) | 2 cases (20%) |
| Coronary alterations (z score) | 4 cases (19%) +1.5 (+1.1 a +2.3) | 6 cases (60%) +3.4 (+2.9 a +5.6) |
| Laboratory | Mean (range) | Mean (range) |
| CPK Mb (vn < 25) | 36 (20-45) | 24 (12-32) |
| Platelets | 128 x 103 (44,000-261,000) | 468 x 103 (387,000-670,000) |
| PCR (vn < 0,5) | 4,5 (1,2-8,6) | 1,8 (0.6-2.2) |
| VSG (vn < 25) | 46 (23-60) | 28 (12-45) |
| Ferritin (vn < 95) | 230 (186-1,380) | 130 (82-156) |
| Serology SARS-CoV-2 | IgM or IgG 12/21 RT-PCR 16/21 | — |
Table 1: Clinical and demographic characteristics.
31 cases were included in the study, 21 cases of COVID-19, and 10 cases of KD. In all cases, an electrocardiogram and/or Doppler echocardiogram were performed, reflecting different findings. 15 of the 21 cases with COVID-19 met the criteria for MIS-C. Of the 10 cases of KD, 75% were classic type and 3 were incomplete type.
Non-specific ST and T-wave alterations were the most frequent changes in the ECG, and among the arrhythmias, there was a patient who recorded sinus pauses (Figure 1) and one case with typical ECG changes for acute pericarditis (Figure 2) with ST-segment elevation which reflects the abnormal repolarization that develops secondary to pericardial inflammation. In the echocardiogram, the most common finding was mild pericardial effusion (Figure 3) and a transient compromise of LV systolic function (Figure 4). Coronary alterations were found in 60% of the patients with KD (Figure 5 and 6), and in only 18% of the cases of COVID-19 related to the so-called multisystem syndrome associated with COVID (MIS-C).
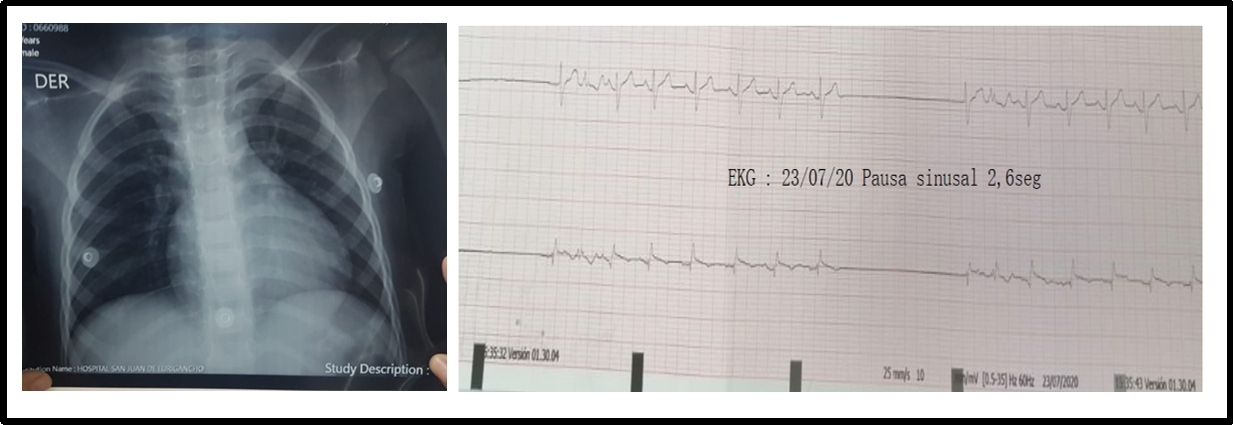
Figure 1: A 3-year-old patient with COVID-19. The X-ray shows a cardiothoracic ratio of 0.52 and ECG with node disease (sinus pauses 2,6 sec).
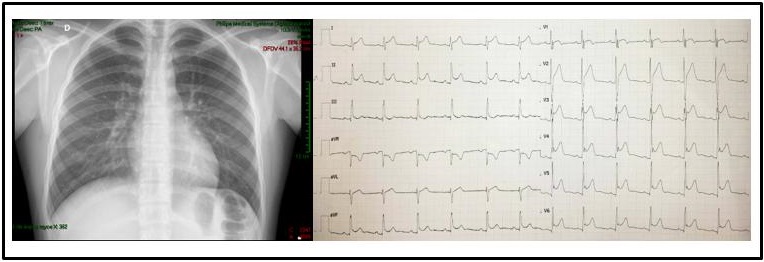
Figure 2: A 14-year-old patient with COVID-19 and acute pericarditis. The X-ray shows a cardiothoracic ratio of 0.50 and ECG with ST-segment elevation.
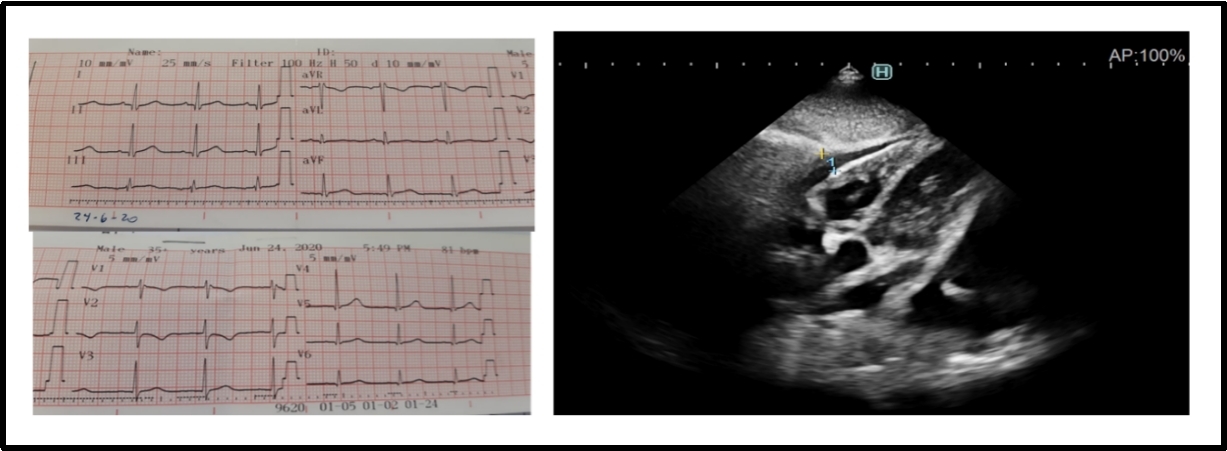
Figure 3: A 6-year-old patient with COVID-19 and acute pericarditis. ECG without significant change in ST-segment and the echocardiogram with mild pericardial effusion.
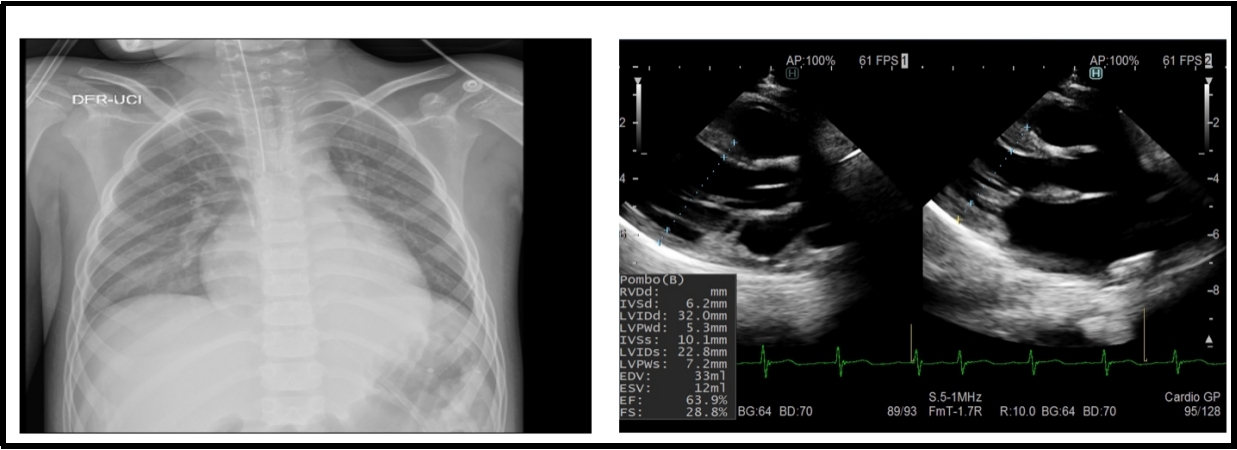
Figure 4: A 9-year-old patient with multisystem syndrome associated with COVID-19. The X-ray shows a cardiothoracic ratio of 0.60 and the echocardiogram with a transient compromise of left ventricular systolic function (FE: 51–63.9%).
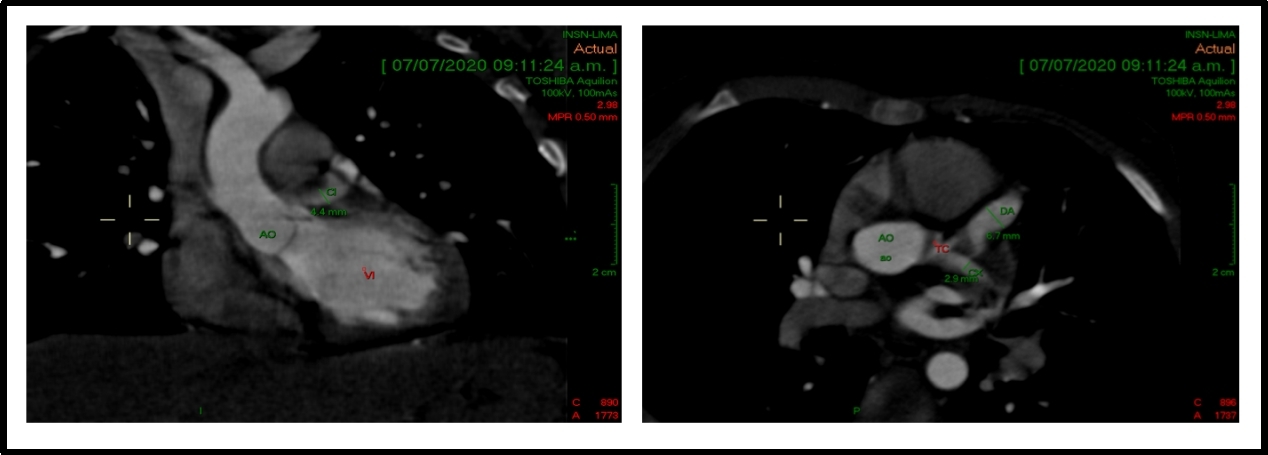
Figure 5: A 2-year-old patient with Kawasaki disease and coronary alterations. A cardiac CT and echocardiogram show a coronary artery aneurysm in LCA (z score +4.7).
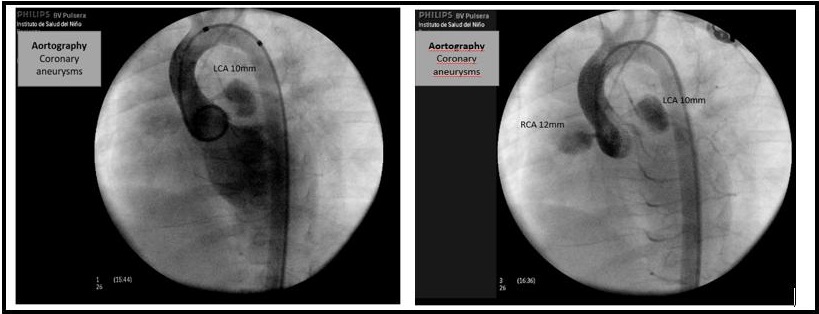
Figure 6: A 3-year-old patient with Kawasaki disease and severe coronary alterations. The aortography shows coronary artery aneurysm of the RCA (right coronary artery) (z score +4.9) and LCA (left coronary artery) (z score +5.6).
In the laboratory abnormalities, thrombocytosis was found in 7 cases (33%) and increased CPK-MB in 8 cases (38%) of the patients with COVID, while thrombocytosis was found in 8 (80%) of the patients with KD.
Regarding the tests for SARS-CoV-2, positive IgM and/or IgG serology was found in 15 patients, and RT-PCR molecular test in 16 patients.
The clinical evolution was mostly favorable and there was no mortality related to any of the diagnoses. In 6 patients with COVID (28.5%), ICU management was required due to myocarditis with heart failure and pulmonary thromboembolism. The average hospitalization stay was 9 days for COVID and 18 days for KD.
Discussion
Our research has focused on the cardiovascular compromise presented by hospitalized pediatric patients during the COVID-19 pandemic in our country.
We studied two groups, the cases of SARS-CoV-2 infection and the cases of KD, and found different clinical evidence of cardiovascular alterations documented from electrocardiograms and echocardiograms.
The electrocardiographic findings reported in patients can vary from normal findings to ST-segment and T-wave abnormalities reflecting pericarditis, myopericarditis, myocardial ischemia, or pulmonary thromboembolism caused by the disease. The echocardiographic findings in these patients may be alterations in the segmental motility of the left ventricle, left and right ventricular systolic dysfunction, pericardial effusion, mitral regurgitation, and in severe cases of MIS-C, the involvement of coronary arteries with dilation and aneurysms is similar to that of KD [13].
In this regard, reports from Europe and North America describe this hyper-inflammatory condition in children related to COVID-19 which presented some characteristics similar to KD, with profound cardiovascular involvement [13]. The Center for Control and Disease Prevention recognized this clinical complex as multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 and published a case definition based on clinical and laboratory criteria [14].
In our work, we found 62% of patients with MIS-C, among the patients with COVID-19, 6 of them presented hemodynamic compromise and required ICU management. The cause was mainly myocarditis with transitory left ventricular dysfunction that required the use of inotropes and one case was diagnosed with bilateral distal pulmonary thromboembolism which evolved favorably. Similar to what has been reported in other publications, cardiovascular compromise in children seems to be more acute, transitory, and of low mortality as compared to adults [15].
Within arrhythmias, although they are described less frequently, we report the case of a 2-year-old patient who developed sinus node disease characterized by significant sinus pauses of up to 2.8 sec in the subacute phase of SARS-CoV infection [2]. Initially, the need for a temporary pacemaker was considered, followed by a permanent pacemaker, but the patient gradually improved over the course of days until complete remission of the pauses occurred in 10–12 days.
The mechanism in COVID-19 of cardiac injury and shock is not clear. One proposed theory is cytokine-mediated myocardial inflammation. The release of cytokines seems to be the main cause of multi-organ dysfunction seen in patients with COVID-19 and may have an impact on myocardial function accompanying the dysfunction of other vital organs [16].
The other hypothesis postulates that myocardial injury is due to direct viral infection causing myocarditis. A report of endomyocardial biopsy in COVID-19 has shown both viral particles and inflammatory infiltrates in the myocardium [17]. In addition, severe hypoxia caused by pneumonia can cause oxidative stress and myocardial injury due to increased myocardial oxygen demand in the presence of a distress syndrome, such as acute respiratory distress syndrome (ARDS).
The virus uses the angiotensin-converting enzyme 2 (ACE2) as a functional receptor for its entry into the cell. This enzyme is a membrane protein expressed in the lung (type 2 pneumocytes), heart, kidney, and intestine and is primarily associated with cardiovascular disease. After the virus enters the cell, the viral RNA is released into the cytoplasm and is transferred into polyproteins and structural proteins where its replication occurs. Later this new protein coating that contains the viral particles fuses with the plasma membrane to release the virus from the cell [18].
The endothelial damage theory could explain the lower cardiovascular compromise in pediatrics since previous endothelial dysfunction can facilitate and increase the inflammatory response to SARS-CoV-2. However, in healthy children, endothelial damage is practically absent. On the other hand, compared to adults, children seem to be less susceptible to COVID-19 due to a reduction in angiotensin-converting enzyme type 2 receptors [19].
The incidence rate in children is ten times lower than in adults, according to MINSA (Ministry of Health) reports [20]. There are already some national studies regarding COVID-19 in the pediatric population [21, 22]. A CDC report in China showed that less than 1% of COVID-19 cases were found in children under 10 years of age. About 90% of the cases were mild to moderate which is why it seems that children are less vulnerable to COVID-19 than adults, but it can also present as a cardiovascular compromise [23].
KD is a self-limited systemic acute vasculitis that occurs more commonly in children between 3 and 5 years old. It was first described in Japan by Dr. Tomosaku Kawasaki in 1967 [24]. Its etiology is unknown. It has also been observed that the disease has a certain seasonal behavior increasing the number of cases and hospitalizations in winter and spring [25]. The two most common sequelae are coronary aneurysms and myocardial infarction. Other complications include myocarditis, pericardial effusion with pericarditis, mitral valvulitis, and coronary artery stenosis. Coronary aneurysms occur in 20–25% of all patients who do not receive treatment with intravenous immunoglobulin [26].
We report 10 cases presented in an unusual period according to the seasonal behavior of KD. The most important cardiac compromise in our patients was the finding of coronary alterations in 60% of them, and pericarditis with mild pericardial effusion in 1 case. A moderate-grade coronary aneurysm (z score > +5) was found in the right and left coronary arteries in two of the cases without evidence of myocardial ischemia or infarction. In general, there was no hemodynamic compromise in any of the cases.
KD outbreaks have been documented in some studies in relation to COVID-19 cases. Although the etiology of KD remains unclear, the role of a viral trigger in some cases has been hypothesized that there are genetically predisposed children, as several viral respiratory agents have been associated with KD, including seasonal coronavirus in some but not in all studies [27].
Comparing MIS-C vs. KD, we found that MIS-C appears to affect older children more (an average of nine years vs. four years, respectively), often with abdominal pain and diarrhea along with common features such as persistent fever. It also appears to affect a higher proportion of black and Asian patients. The blood tests also show different results. The MIS-C shows more markers of inflammation and cardiac enzymes as well as thrombocytopenia, unlike the thrombocytosis that usually accompanies KD [11]. Most of these findings were described in both groups of our patients.
Currently, no recommendations have been established on the treatment of myocardial injury and inflammation related to COVID-19. The use of steroids and IVIG have been described for treatment in adults with myocarditis due to COVID-19 with unclear benefits [28]. It is the same scheme management received by the patients in our study.
Conclusion
Pediatric patients with COVID-19 and KD may present various cardiovascular alterations. So, in the face of clinical suspicion, the early performance of an electrocardiogram and/or echocardiogram will provide a timely diagnosis of complications optimizing their management.
The clinical picture in both the groups presented some similar characteristics, mainly centering on coronary compromise, which was greater in KD, and in the evolution, a greater hemodynamic compromise was evident in the cases of SARS-CoV-2 infection in relation to multisystem inflammatory syndrome but without associated mortality.
Long-term cardiovascular complications in children with COVID-19 are not yet known, so follow-up studies should be designed, approved, and carried out.
Ethical Considerations
The database was registered anonymously, using codes as a way of characterizing the cases, in order to avoid the identification of patients and respect the reliability of the data.
Funding
Conflicts of Interest
The authors declare that they have no conflict of interest in any of the stages of development of this article.
References
- Ministerio de Salud. Alerta epidemiológica ante el incremento de casos de COVID-19 en el Perú. 2020.
- Ministerio de salud. Lima, MINSA; 2020.
- Beroukhim RS, Friedman KG. Children at Risk: Multisystem Inflammatory Syndrome and COVID-19. J Am Coll Cardiol Case Rep. 2020;2(9):1271-274.
- Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39(5):355-68.
- Joshi K, Kaplan D, Bakar A, et al. Cardiac Dysfunction and Shock in Pediatric Patients With COVID-19. J Am Coll Cardiol Case Rep. 2020;2(9):1267-270.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-69.
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663-665.
- Pediatric Intensive Care Society. PICS Statement Regarding Novel Presentation of Multi-System Inflammatory Disease.
- Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. 2020;126(10):1443-455.
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory Shock in Children During COVID-19 Pandemic. Lancet. 2020;395(10237):1607-608.
- Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr. 2020;10(6):537-40.
- Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-Related Paediatric Inflammatory Multisystem Syndrome, an Epidemiological Study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):2001010.
- Ebina-Shibuya R, Namkoong H, Shibuya Y, et al. Multisystem Inflammatory Syndrome in Children (MIS-C) with COVID-19: Insights from Simultaneous Familial Kawasaki Disease Cases. Int J Infect Dis. 2020;97:371-73.
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. Morbidity and Mortality Weekly Report. 2020.
- Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-24.
- Channappanavar R, Perlman S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin Immunopathol. 2017;39(5):529-39.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and Viral Sepsis: Observations and Hypotheses. Lancet. 2020;395(10235):1517-520.
- Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185-92.
- Li X, Geng M, Peng Y, et al. Molecular Immune Pathogenesis and Diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102-08.
- Centro Nacional de Epidemiología, Prevención y Control de Enfermedades – Sala Situacional de COVID-19.
- Llaque P. Infección por el nuevo coronavirus 2019 en niños. Rev Peru Med Exp Salud Publica [nternet]. 2020;37(2):335-40.
- De Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, et al. Síndrome Inflamatorio Multisistémico Asociado a COVID-19 en Niños: Serie de Casos en un Hospital Pediátrico de Perú. Rev Peru Med Exp Salud Publica [nternet]. 2022;37(3):559-65.
- Sun D, Li H, Lu XX, et al. Clinical Zvere Pediatric Patients with Coronavirus Disease 2019 in Wuhan: a Single Center’s Observational Study. World J Pediatr. 2020;16(3):251-59.
- Dietz SM, van Stijn D, Burgner D, et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176(8):995-1009.
- Ozeki Y, Yamada F, Kishimoto T, et al. Epidemiologic Features of Kawasaki Disease: Winter versus Summer. Pediatr Int. 2017;59(7):821-25.
- Yılmazer MM, Özdemir R, Meşe T, et al. Kawasaki Disease in Turkish Children: a Single Center Experience with Emphasis on Intravenous Immunoglobulin Resistance and Giant Coronary Aneurysms. Turk J Pediatr. 2019;61(5):648-56.
- Chang LY, Lu CY, Shao PL, et al. Viral infections Associated with Kawasaki Disease. J Formos Med Assoc 2014;113(3):148-54.
- Nguyen AA, Habiballah SB, Platt CD, et al. Immunoglobulins in the Treatment of COVID-19 Infection: Proceed with Caution! Clin Immunol. 2020;216:108459.
![]() *1, Taipe F
*1, Taipe F![]() 1, Nario V
1, Nario V![]() 1 and Culqui K
1 and Culqui K![]() 2
2
 KD criteria is representative of the AHA. Classic KD is diagnosed when the patient has a fever for more than 5 days and at least 4 of the following clinical characteristics – bilateral conjunctival injection, changes in the lips and oral cavity, cervical adenopathy, changes in the extremities, and polymorphous rash. If the patient has few clinical findings but abnormalities in the coronary arteries are found on the echocardiogram, then the diagnosis can be made, since the clinical features tend to appear sequentially.
KD criteria is representative of the AHA. Classic KD is diagnosed when the patient has a fever for more than 5 days and at least 4 of the following clinical characteristics – bilateral conjunctival injection, changes in the lips and oral cavity, cervical adenopathy, changes in the extremities, and polymorphous rash. If the patient has few clinical findings but abnormalities in the coronary arteries are found on the echocardiogram, then the diagnosis can be made, since the clinical features tend to appear sequentially.




