Introduction: Systemic amyloidosis AL is an unusual disease characterized by a heterogeneous presentation.
Clinical Case: We present a case of systemic amyloidosis AL with unusual cardiac, mesenteric, renal, peritoneal, and gastrointestinal involvement, in which the diagnostic hypothesis was formulated on the basis of problem-solving and ultrasound technique (abdomen, lung, heart ultrasound).
Conclusion: This case shows that multi-organ ultrasound, associated with problem-solving, may be very helpful in the diagnosis of systemic diseases, even unusual ones, avoiding a long diagnostic interval length.
Systemic amyloidosis AL is an unusual disease characterized by a heterogeneous presentation. Therefore, the combination of problem-solving and multi-organ ultrasonography is often a valid aid to get to the diagnosis of a long-been misunderstood disease. Moreover, early diagnosis can improve the prognosis and reduce organ dysfunction.
We present a case of systemic amyloidosis AL with unusual cardiac, mesenteric, peritoneal, and gastrointestinal involvement.
A 73-year-old woman was referred to our emergency room by a pulmonologist due to recurrent relapses of pleural effusion in the last year.
In the emergency room, the patient underwent chest x-ray (bilateral pleural effusion, larger on the left); blood tests (Hb 8.7 g/dL, creatinine 1.74 mg/dL, glomerular filtration rate: GFR 29 mL/min, CRP: c-reactive protein 30 mg/L); hemogasanalysis (pH 7.4, PCO2 44 mmHg, PO2 63 mmHg, SaO2 91%, HCO3 18 mEq/L).
Remote medical history showed congenital deaf-mute, bronchial asthma and chronic obstructive pulmonary disease (COPD), hypertension, dyslipidemia, multinodular pretoxic goiter, and previous uterine and colon polyps removal.
Recent medical history showed that 9 months before, the patient was admitted to another hospital unit for acute bronchopneumonia with COPD, associated with transient acute respiratory failure. In this hospitalization, bilateral pleural effusion (larger on the left) was subjected to thoracentesis (analysis of pleural fluid showed exudate, non-neoplastic cytology).
Eight months before, the patient was admitted to another special unit for significant weight loss (19 kg in 3 months) with hyporexia. The diagnostic hypothesis was gastric neoplasia; therefore, the patient was subjected to esophagogastroduodenscopy (EGD) with biopsy. Two gastric biopsies were negative for neoplastic localization. Subsequently, an ecoendoscopy showed gastric wall thickened and an early stage of chronic pancreatitis. The abdomen computed tomography was positive for diffuse effusion in the mesenteric adipose tissue and in the peritoneum, thickening of antropyloric gastric wall and the small intestine tract, Wirsung dilatation, rounded formation of 5.7 cm of the spleen, with enhancement in the arterial phase and contrast homogenization in subsequent stages of examination. EGD was reproposed in order to repeat a biopsy, but the patient refused the test.
In our medicine, we performed
Blood tests: chronic renal failure at stage III/IV GFR (29–30 mL/min), proteinuria was 3.67 mg per 24 h predominantly glomerular, monoclonal component (k free light chains), k free light chain concentration (FLC) was 4850 mg/L, ratio (FLCR) 69.8, differential (dFLC) 4780 mg/L, the elevation of N-terminal pro natriuretic peptide type B (proBNP) (5348 ng/L), beta-2 microglobulin increased, Bence-Jones urine positive, Quantiferon indeterminate.
Lung ultrasonography showed massive left pleural effusion and abundant right pleural effusion subjected to thoracentesis (1800 cc), with rapid reformation. Analysis of pleural fluid showed transudate; microbiological and cytological tests were negative.
We have formulated the following clinical problems: weight loss (19 kg in 3 months), dyspnea, hyporexia in a patient with gastric wall thickening, widespread involvement of the mesenteric adipose tissue and the peritoneum, heart failure secondary to hypertrophic cardiomyopathy, monoclonal component, chronic renal failure associated with proteinuria.
We hypothesized a systemic pathology: a lymphoproliferative disease, a storage disease, or an infectious disease (e.g., tuberculosis).
The patient underwent multi-organ ultrasonography, showing in the epigastric scan (Figure 1) macroscopic thickening of the interventricular septum (septum 2.3 cm), severe thickening of the left and right ventricles walls, biatrial dilatation and irrelevant pericardial effusion (1.1 cm). The cardiac finding was very suggestive of amyloidosis, so we required an echocardium.
Abdomen ultrasound show diffuse amorphous slightly hyperechoic material (including peritoneum, mesentery, perisplenic region, and around the stomach wall) associated with abdominal effusion (Figure 2, 3, and 4). In another setting, the abdomen ultrasound showed hepatomegaly, and splenomegaly, associated with the hilar hyperechoic area of about 5 cm (without wash-in in contrast-enhanced ultrasound). Lung ultrasonography confirmed bilateral pleural effusion. Because of systemic involvement and monoclonal component, we hypothesized an AL amyloidosis.
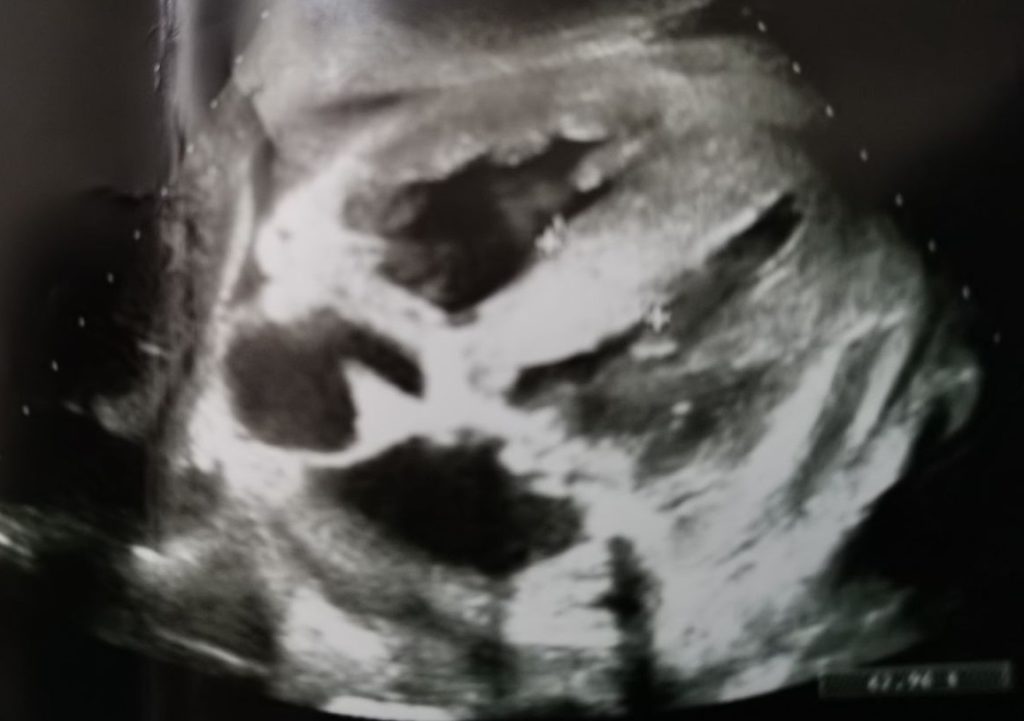
Figure 1: Epigastric scan. Macroscopic thickening of the interventricular septum, severe thickening of the left and right ventricles walls, biatrial dilatation, and irrelevant pericardial effusion.
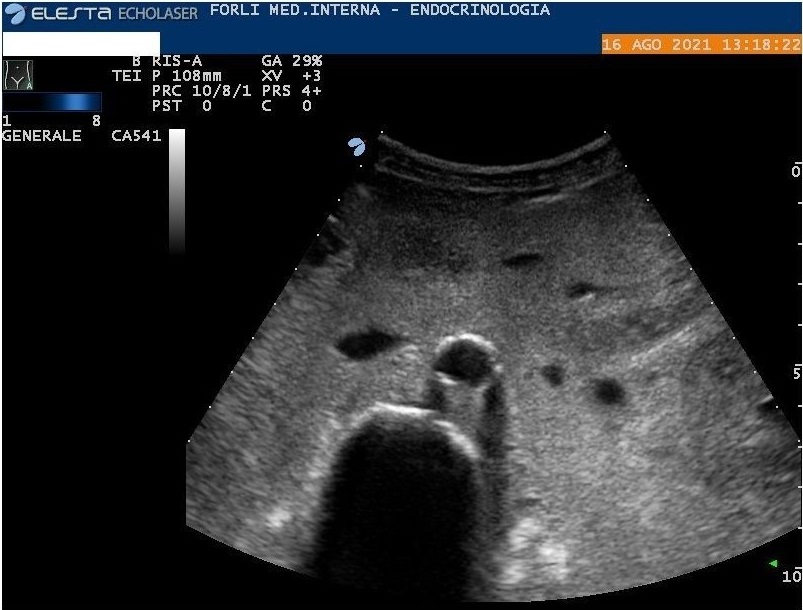
Figure 2: Abdomen ultrasound. Diffuse amorphous slightly hyperechoic material in peritoneum and mesentery.
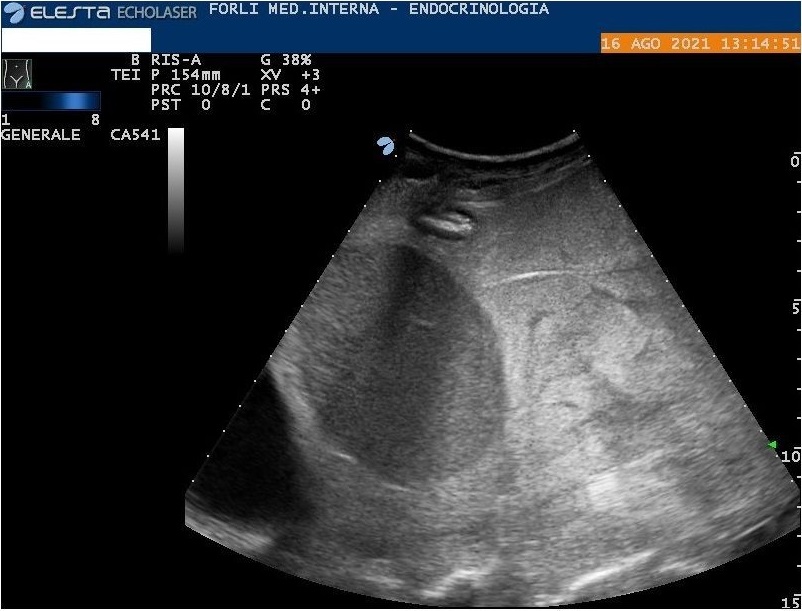
Figure 3: Abdomen ultrasound. Diffuse amorphous slightly hyperechoic material in the perisplenic region.
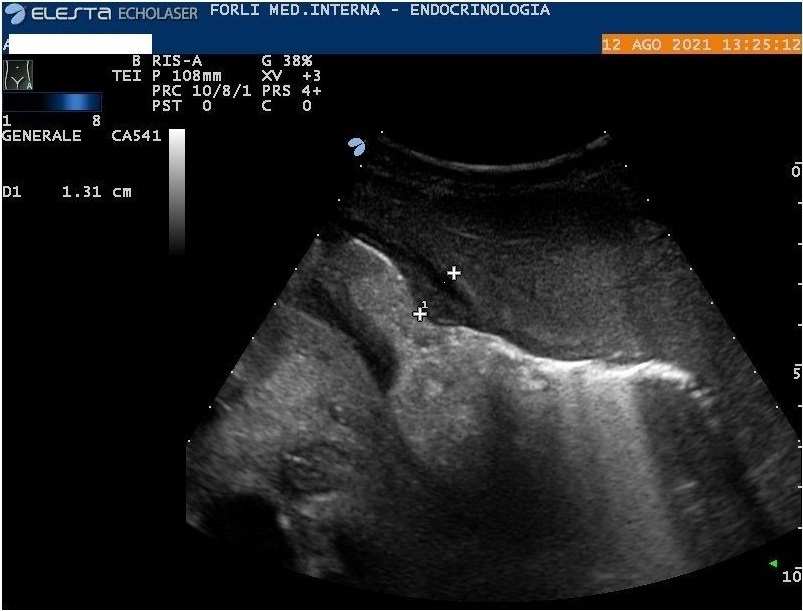
Figure 4: Abdominal ultrasound. Amorphous material in the abdomen, around the stomach wall.
To test the diagnostic hypothesis, we performed an echocardium (increased ventricular thickness and preserved ejection fraction compatible with storage disease); a periumbilical fat biopsy (Congo Red staining negative for amyloid deposits); a 99 Tc bone scan scintigraphy (negative for cardiac uptake: score 0), reasonably excluding amyloidosis transthyretin related. FDG-positron emission tomography was positive for gastric wall uptake. Finally, in our Pathological Anatomy Institute, gastric mucosal biopsies were reanalyzed, and Congo Red staining was positive for amyloid deposits.
Bone marrow biopsy and bone marrow aspirate showed plasma cellular clone infiltration of 30%.
We concluded a differentiated plasma cell myeloma associated to AL amyloidosis with cardiac, renal, peritoneal, mesenteric, and gastrointestinal involvement.
Evolution
We treated the patient with a continuous infusion of furosemide and water restriction; however hypertensive crisis and hematuria occurred and pleural effusion appeared unresponsive to therapy. This case was discussed with nephrologists, cardiologists, and pulmonologists; we started the therapy with furosemide, canrenone, metolazone, inotropic (dopamine), albumin, and finally, a new thoracentesis is performed (1600 cc). Subsequently, a partial clinical response occurred.
Therefore, the patient was transferred to the Hematology Unit and myeloma treatment was started. Unfortunately, she had a quick bad progression until death because of the advanced stage of renal and cardiac involvement.
In this case, AL amyloidosis is associated with systemic involvement and usual (heart, renal, gastrointestinal tract) and unusual (omentum and peritoneum) localization [1, 2]. Indeed, widespread involvement of the mesenteric tissue and the peritoneum of AL amyloidosis is extremely rare in the literature, to the best of our knowledge.
As a confirmation of this, we performed systematic research on PubMed (Mesh term “Mesentery” AND “Peritoneum” AND “Amyloidosis”). We carried out extended web searches, using the following keywords: “Peritoneum”, “Mesentery”, and “Amyloidosis”. In the last 20 years, we have obtained only 15 cases and four of them were excluded since they were not related to AL amyloidosis [3–13]. Amyloid depositions can often mimic both neoplastic and non-neoplastic conditions, and this diagnosis should be considered a possible etiology of deposit of amorphous material in the abdomen associated with ascites.
Currently, multi-organ ultrasound in the literature is extensively described in thromboembolism, in the assessment of dyspnea, and, limited to systemic diseases, in Covid 19 infection [14–16].
This case shows that multi-organ ultrasound, associated with problem-solving, may be very helpful in the diagnosis of systemic diseases, even unusual ones or associated with non-specific clinical aspects, avoiding a long diagnostic interval length with improvement in the prognosis of patients.




