Abstract
Background: Various irrigating solutions have been used during pulpectomy and root canal procedures. However, each one of them has its own demerits. As a result, there is a quest for novel bioactive materials that are safer and biodegradable.
Aim: To evaluate and compare the antimicrobial efficacy of pomegranate, tulsi, garlic, and liquorice as a root canal irrigant against Enterococcus faecalis.
Methodology: 0.2% chlorhexidine, pomegranate, tulsi, garlic, and liquorice extracts with different concentrations of 5%, 25%, 50%, 100% and fresh stains of Enterococcus faecalis (ATCC 29212) were used. The agar well diffusion method was performed to evaluate and compare the antibacterial efficiency of all the groups.
Results: The results were tabulated and statistically analyzed using analysis of variance (ANOVA). The mean zone of inhibition was highest for Group A (0.2% chlorhexidine), i.e., 18.36 mm, followed by Group E (liquorice extract), i.e., 17.40 mm, Group C (tulsi extract), i.e., 16.43 mm, and Group B (pomegranate extract), i.e., 14.55 mm.
Conclusion: All four herbal products (pomegranate, tulsi, liquorice, and garlic) possess antimicrobial activity against Enterococcus faecalis, with liquorice and tulsi extract having maximum effect, followed by pomegranate and garlic extract having minimum antimicrobial activity.
Keywords
agar diffusion test, chlorhexidine, Enterococcus faecalis, pomegranate extract, zone of inhibition
Abbreviations
ANOVA: analysis of variance, ESP: enterococcal surface proteins, BHI: brain heart infusion, CLSI: Clinical Laboratory Standard Institute guidelines
Introduction
Primary endodontic infections are caused by oral microorganisms, which are usually opportunistic pathogens that may invade a root canal containing necrotic tissue and establish an infectious process [1]. The number of facultative anaerobic bacteria increases when the root canal remains infected for long periods. The major objective of root canal treatment is to disinfect the entire root canal system. Although cleaning, shaping, and the use of antimicrobial medicaments are effective in reducing the bacterial load, some bacteria do remain behind and multiply, causing re-infection of the canal. Residual pulpal tissue, bacteria, and dentine debris may persist in the irregularities of root canal systems, even after meticulous mechanical preparation. Therefore, irrigant solutions should be used in combination with canal preparation [2].
Root canal irrigants are used during chemo-mechanical procedures not only as antimicrobial agents but also to flush out loose debris, lubricate the dentinal walls, and dissolve organic compounds in the canal. Several chemicals and therapeutic agents have been used to disinfect the root canal. The most effective among them are sodium hypochlorite, chlorhexidine, and calcium hydroxide, which possess varying degrees of antibacterial activity [2–6].
Most of the bacteria in an endodontic infection are “strict anaerobes”. Enterococcus faecalis is the most commonly isolated species from the canals of teeth presenting post-treatment diseases. They account for up to 77% of therapeutic failures [3]. Enterococci are gram-positive cocci that can occur singly, in pairs, or as short chains. They are facultative anaerobes, possessing the ability to grow in the presence or absence of oxygen. Its high prevalence in cases with post-treatment disease associated with virulence factors (aggregation substance, enterococcal surface proteins (ESP), gelatinase, cytolysin toxin, extracellular superoxide production, capsular polysaccharides, antibiotic resistance determinant) can facilitate the adherence of host cells and extracellular matrix, tissue invasions, immunomodulation effect, and cause toxin-mediated damage [4–10].
The use of plants and plant products as medicines could be traced as far back as the beginning of human civilization. Considering the ineffectiveness, potential side effects, and safety concerns of synthetic drugs, the herbal alternatives for endodontic usage might prove to be advantageous [2].
Pomegranate (Punica granatum Linn) is a potent antioxidant with anti-carcinogenic and anti-inflammatory properties. The plant is known to have both medicinal and nutritional benefits. In Ayurvedic medicine, the pomegranate is considered “a pharmacy unto itself” and as a remedy for diabetes in the Unani system of medicine practiced in the Middle East and India. Various components of the plant, such as the leaves, flowers, roots, bark, and extracts of the fruit, including the pericarp, seed oil, and juice, have been used [8].
Tulsi, scientific name “Ocimum sanctum Linn”, is a sacred, holy Hindu Laxmi Goddess Basil, a medicinal plant. It is a member of the mint or Labiatae family from India. Medicine from leaves, seeds, and stems is commonly used for cold, influenza, H1N1 (swine flu), hepatitis, bronchitis, stress, cancer, headache, heart diseases, malaria, digestive disorders, etc. It is a powerful antioxidant, anti-fungal, antibacterial, and anti-inflammatory agent [7].
Garlic (Allium sativum L.) has many properties, such as antimicrobial, antiplatelet, antithrombotic, and anticancer activity. The common organisms inhibited by garlic include Streptococcus mutans, Staphylococcus aureus, and Escherichia coli [1].
Liquorice (Glycyrrhiza glabra L.) is a native of south-east Europe and south-west Asia, including Iran. The root of this plant has several useful pharmacological properties, such as anti-inflammatory, antiviral, antimicrobial, and anticancer activities in addition to immunomodulatory, hepatoprotective, and cardioprotective effects [11–14].
In dentistry, because of the cytotoxic reactions of most of the commercial intracanal medicaments used and their inability to eliminate bacteria from dentinal tubules, there is a quest for novel bioactive materials/ herbal alternatives that are popular, have increased shelf life, and are less toxic. Therefore, the present study was designed to explore the antimicrobial efficacy of pomegranate, tulsi, garlic, and liquorice extracts as a root canal irrigant against Enterococcus faecalis using the agar diffusion method.
The objectives of the study are as follows:
- To evaluate the antimicrobial efficacy of pomegranate, tulsi, garlic, and liquorice as a root canal irrigant against Enterococcus faecalis.
- To evaluate the minimum inhibitory concentration of all four extracts at different concentrations on Enterococcus faecalis.
- To compare the antimicrobial efficacy of pomegranate, tulsi, garlic, and liquorice as a root canal irrigant against Enterococcus faecalis with 0.2% chlorhexidine.
Materials and Methods
2.1 Source of data
The bacterial stock culture of Enterococcus faecalis strain (ATCC 29212) was obtained, and the extracts of pomegranate, tulsi, garlic, and liquorice were prepared from Bapuji Pharmacy College, Davangere, Karnataka, India.
2.2 Pomegranate fruit extract preparation
Fresh ripe pomegranate fruits were obtained from the local market. After washing, the peel/rind (pericarp) was removed. A total of 100 g of cleaned pomegranate pulp and chloroform water, i.e., 2.5 ml of chloroform in 1000 ml of purified water (Indian Pharmacopoeia), was added to a juicer and crushed. The mixture was filtered using a double filter paper, and the supernatant was then centrifuged at 8,000 rpm for 40 min.
2.3 Tulsi extract preparation
Fresh tulsi leaves were washed and prepared for extraction. A total of 100 g of cleaned tulsi leaves and chloroform water, i.e., 2.5 ml of chloroform in 1000 ml of purified water (Indian Pharmacopoeia), was added to a juicer and crushed. The mixture was filtered using a double filter paper, and the supernatant was then centrifuged at 8,000 rpm for 40 min.
2.4 Garlic extract preparation
Fresh garlic was obtained from a retail spice seller, and taxonomic identification was done. A total of 100 g of cleaned garlic bulbs and chloroform water, i.e., 2.5 ml of chloroform in 1000 ml of purified water (Indian Pharmacopoeia), was added to a juicer and crushed. The mixture was filtered using a double filter paper, and the supernatant was then centrifuged at 8,000 rpm for 40 min.
2.5 Liquorice extract preparation
Dry powder standardized to contain 20% glycyrrhizinic acid. A total of 100 g of liquorice powder and chloroform water, i.e., 2.5 ml of chloroform in 1000 ml of purified water (Indian Pharmacopoeia), was added to a juicer and crushed. The mixture was filtered using a double filter paper, and the supernatant was then centrifuged at 8,000 rpm for 40 min.
2.6 Agar diffusion test
The standard strain of Enterococcus faecalis (ATCC 29212) was grown on brain heart infusion (BHI) broth overnight, and turbidity was adjusted to 0.5 McFarland scale to obtain a cell density of 1.5 × 108 bacteria/mL and inoculated in Mueller-Hinton agar plates. Inoculation was performed by using a sterile swab that was brushed across the media. Four round wells measuring about 4 mm deep and 8 mm in diameter were punched using a sterile stainless-steel template, and they are numbered as 1, 2, 3, and 4 consecutively for the different concentrations used for each test group. As a control, a 0.2% chlorhexidine gluconate solution was used for the evaluation.
Five groups were made and named as A, B, C, D, and E for the five testing agents used, including a control, consisting of 15 inoculation agar plates in each group. Group A was allocated for chlorhexidine (control), Group B for pomegranate extract, Group C for tulsi extract, Group D for garlic extract, and Group E for liquorice extract.
After making serial dilutions of each extract and four round wells in each agar plate, 50 μL of a specific concentration of each extract was dispensed into each well using a sterile micropipette. This was done in triplicate for every concentration so as to overcome any inadvertent technical errors. This was done for each group in the same way. All agar plates were then incubated at 37ºC for 24 h, according to Clinical Laboratory Standard Institute guidelines (CLSI). Following 24 h of incubation at 37ºC, zones of inhibition (that is, areas where no growth of bacteria is present) were examined around each well. They appeared as a clear, circular halo surrounding the wells. Diameters of the bacterial growth inhibition zones or halos were measured using a Hi antibiotic zone scale in millimeters and this represented the inhibition value.
Results
The results were subjected to statistical analysis by applying analysis of variance (ANOVA) and post hoc Tukey HSD tests for multiple comparisons. Mean values of the zone of bacterial inhibition (mm) of five medicaments at four different concentrations and control chlorhexidine after 24 h. The mean zone of inhibition for positive control, that is Group A (0.2% chlorhexidine), was 18.36 mm, with which all other values were compared (Table 1).
On applying one-way ANOVA, a statistically significant difference was seen between the zone of inhibition of five samples within groups and between groups, i.e., P < 0.001. Post hoc Tukey HSD tests were applied to make multiple comparisons between different groups, such as Group A (0.2% chlorhexidine), Group B (pomegranate), Group C (tulsi), Group D (garlic), and Group E (liquorice), with different concentrations of 5%, 25%, 50%, and 100%. The mean zone of inhibition was highest for Group A (0.2% chlorhexidine), i.e., 18.36 mm. Group B (pomegranate extract) with different concentrations of 5%, 25%, 50%, and 100% was 10.18 mm, 12.20 mm, 13.55 mm, and 14.55 mm, respectively (Graph 1). Group C (tulsi extract) with different concentrations of 5%, 25%, 50%, and 100% was 10.54 mm, 12.46 mm, 13.11 mm, and 16.43 mm, respectively (Graph 2). The value was least for the control Group D (garlic extract) with different concentrations of 5%, 25%, 50%, and 100% which were 0.22 mm, 0.53 mm, 1.06 mm, and 1.40 mm, respectively (Graph 3). Group E (liquorice extract) with different concentrations of 5%, 25%, 50%, and 100% was 12.24 mm, 13.3 mm, 16.02 mm, and 17.40 mm, respectively (Graph 4).
| | N | Mean ± Std. deviation | Minimum | Maximum | Class interval 95% | P value |
| 0.2% Chlorhexidine (control) | 15 | 18.360 ± .3602 | 17.7 | 18.9 | 18.200-18.600 | 0.000 < 0.05 (S) |
| Pomegranate (5%) | 15 | 10.180 ± .5493 | 8.9 | 10.8 | 9.800-10.600 | 0.000 < 0.05 (S) |
| Pomegranate (25%) | 15 | 12.207 ± .8548 | 10.0 | 13.7 | 12.000-12.500 | 0.000 < 0.05 (S) |
| Pomegranate (50%) | 15 | 13.580 ± .2396 | 13.0 | 13.9 | 13.500-13.800 | 0.000 < 0.05 (S) |
| Pomegranate (100%) | 15 | 14.553 ± .3357 | 13.9 | 15.0 | 14.400-14.800 | 0.000 < 0.05 (S) |
| Tulsi (5%) | 15 | 10.547 ± .1807 | 10.3 | 11.0 | 10.400-10.600 | 0.000 < 0.05 (S) |
| Tulsi (25%) | 15 | 12.467 ± .3109 | 11.8 | 12.9 | 12.400-12.700 | 0.000 < 0.05 (S) |
| Tulsi (50%) | 15 | 13.113 ± .3871 | 12.5 | 13.9 | 12.900-13.500 | 0.000 < 0.05 (S) |
| Tulsi (100%) | 15 | 16.433 ± .3244 | 16.0 | 16.9 | 16.200-16.800 | 0.000 < 0.05 (S) |
| Garlic (5%) | 15 | .225 ± .0537 | .1 | .3 | .210-.250 | 0.000 < 0.05 (S) |
| Garlic (25%) | 15 | .534 ± .0800 | .4 | .7 | .500-.600 | 0.000 < 0.05 (S) |
| Garlic (50%) | 15 | 1.060 ± .3225 | .5 | 1.9 | 1.000-1.200 | 0.000 < 0.05 (S) |
| Garlic (100%) | 15 | 1.407 ± .4008 | .5 | 1.9 | 1.200-1.700 | 0.000 < 0.05 (S) |
| Liquorice (5%) | 15 | 12.240 ± .5152 | 11.1 | 12.9 | 12.000-12.600 | 0.000 < 0.05 (S) |
| Liquorice (25%) | 15 | 13.347 ± .4853 | 12.0 | 13.9 | 13.300-13.600 | 0.000 < 0.05 (S) |
| Liquorice (50%) | 15 | 16.027 ± .4964 | 15.0 | 16.8 | 16.000-16.200 | 0.000 < 0.05 (S) |
| Liquorice (100%) | 15 | 17.407 ± .3081 | 17.0 | 18.0 | 17.100-17.600 | 0.000 < 0.05 (S) |
Table 1: Mean values of zone of bacterial inhibition (mm) of four medicaments at four different concentrations and control chlorhexidine after 24 h.
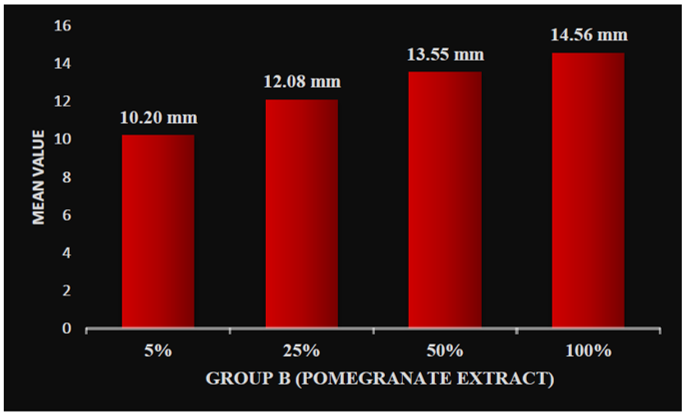 Graph 1: Zone of inhibition at different concentrations of group B (pomegranate) extract.
Graph 1: Zone of inhibition at different concentrations of group B (pomegranate) extract.
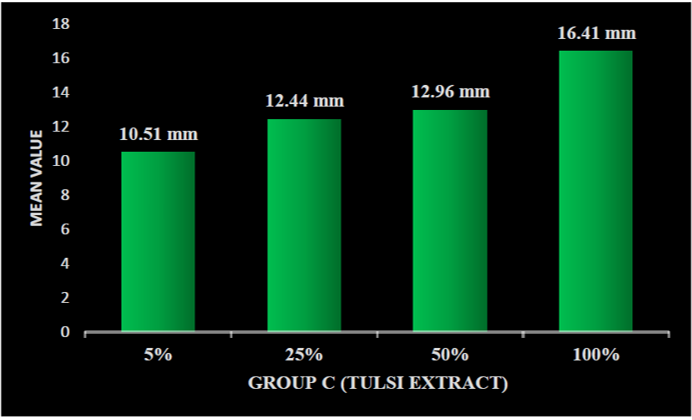 Graph 2: Zone of inhibition at different concentration of group C (tulsi) extract.
Graph 2: Zone of inhibition at different concentration of group C (tulsi) extract.
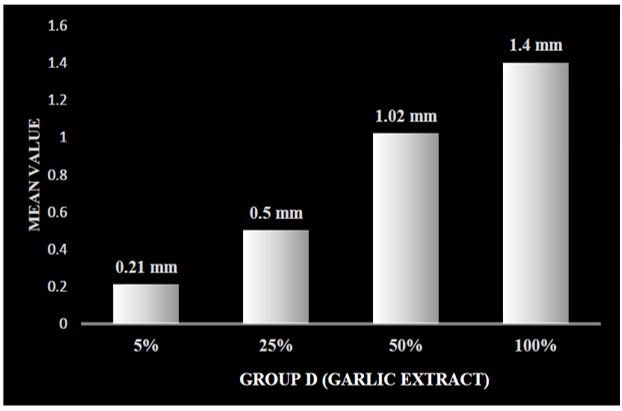 Graph 3: Zone of inhibition at different concentrations of group D (garlic) extract.
Graph 3: Zone of inhibition at different concentrations of group D (garlic) extract.
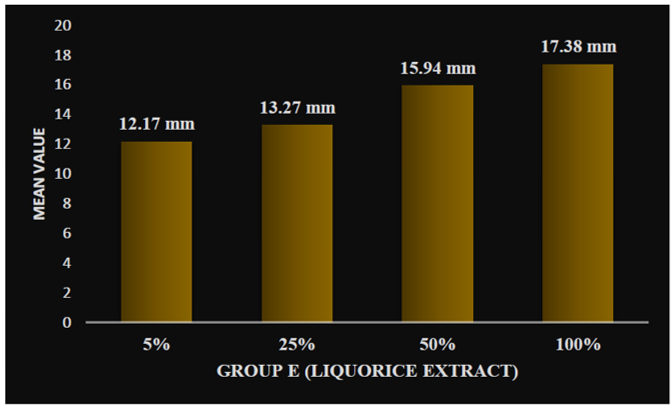 Graph 4: Zone of inhibition at different concentrations of group E (liquorice) extract.
Graph 4: Zone of inhibition at different concentrations of group E (liquorice) extract.
Discussion
Complete debridement and effective disinfection of the root canal space are important prerequisites for achieving long-term success of nonsurgical endodontics. It is known that elimination of microorganisms is critical for the management of pulpal diseases. Chemo-mechanical instrumentation reduces the majority of infecting bacteria, but retention of microorganisms within the dentinal tubules is thought to be a source of persistent endodontic infection. The use of an intracanal irrigant helps in the elimination of bacteria that remain even after cleaning and shaping, thereby making the environment conducive for periapical tissue repair [12–18].
Various chemicals are used to disinfect the root canal. Sodium hypochlorite (NaOCl), chlorhexidine, and normal saline are most commonly used root canal irrigants [2]. Owing to the potential side effects, safety concerns, and ineffectiveness of conventional allopathic formulations, consumption of preparations from medicinal plants has increased over the last few decades due to their high antimicrobial activity, biocompatibility, anti-inflammatory, and antioxidant properties [19, 20]. Currently, the use of natural extracts in dentistry has gained importance among patients and endodontic professionals as the shift is toward natural health remedies [21–23]. The medicinal properties of herbs are due to the presence of different complex chemical substances, which are secondary metabolites such as flavonoids, terpenoids, tannins, glycosides, and alkaloids [5–9]. The use of a biocompatible intracanal medicament possessing antimicrobial properties between appointments may reduce or eliminate bacteria in the root canal system [23].
The present in vitro investigation for the newer antibacterial bioactive compounds targeted on the unexplored folk medicinal plants like pomegranate, tulsi, liquorice, and garlic against Enterococcus faecalis using the agar diffusion method with BHI agar plates. This method has been used by other researchers as well to test the antimicrobial capability of root canal irrigants [21–25]. This methodology demonstrates action against the planktonic forms of the micro-organisms, which is important for an initial screening study [24]. However, its limitations include pH of the substrate, incubation period, and diffusion capacity of the drug, which have an effect on the activity of the test materials. However, evidence also suggests that agar diffusion tests show good correlation with other antimicrobial susceptibility tests [10]. Also, the duration of effectiveness of the drug, temperature, contamination, and possible leakage of the agent into the mouth must be considered whilst working in vivo. BHI agar plates were used in this study as they provide favorable media for the growth of Enterococcus faecalis and are justified by their use in various studies [25–28].
Enterococcus faecalis, a gram-positive facultative anaerobe, was chosen as the primary test organism because it is the dominant species recovered in failed endodontic cases [29].
Enterococcus faecalis is the “star survivor” in the root canal. It has gained significance in recent years due to the mounting resistance to antimicrobial agents [26]. It is also demonstrated to possess the ability to multiply after standard chemo-mechanical procedures. In addition, they may survive even under unusual environmental stresses, such as low nutrient availability, and may be extremely resistant to medications used during the root canal therapy. The use of Enterococcus faecalis in this study is justified because of its reported resistance to chemo-mechanical procedures, it’s supposed involvement in endodontic failures, and because it is relatively easy to culture and manipulate [28–30].
The results of the study showed that after incubation for 24 h, the Group A (0.2 % chlorhexidine) showed the highest zone of bacterial inhibition in all the 15 plates, followed by Group E (liquorice), followed by Group C (tulsi), followed by Group B (pomegranate), and Group D (garlic) had negligible zone of bacterial inhibition in all the 15 plates.
Group A (0.2% chlorhexidine) had a mean value of zone of bacterial inhibition as 18.36 mm, Group E (liquorice) had a mean value of 17.40 mm (at 100% concentration), Group C (tulsi) gave a mean value of 16.43 mm (at 100% concentration), Group B (pomegranate) gave a mean value of 14.55 mm (at 100% concentration), and Group D had very low zone of bacterial inhibition of 1.4 mm.
Liquorice extract (100% concentration) showed antimicrobial activity against Enterococcus faecalis, as shown by the zones of bacterial inhibition at 24 h. However, the antibacterial activity was significantly less when compared to that of 0.2% chlorhexidine. This could be due to the fact that a crude and dry powder of liquorice was used in the study. The extract also might not have diffused well in the agar media compared to 0.2% chlorhexidine. Moreover, a more purified extract, or extracts using other solvents, could have yielded better results.
The antimicrobial effect of liquorice extract against Enterococcus faecalis may be related to the glycyrrhizin and other saponins. The mode of action of the antibacterial effects of saponins seems to involve membranolytic properties, rather than simply altering the surface tension of the extracellular medium, thus being influenced by microbial population density. The flavonoid content of liquorice extract is also a strong inhibitor of oxygen consumption in bacterial cells, the site of inhibition being between the cytochrome Q and cytochrome C in the bacterial respiratory electron transport chain [23].
In this study, tulsi extract had a mean zone of inhibition at 100% concentration of 16.43 mm, which was in agreement with that of Subbiya et al. [28]. This study revealed that tulsi leaf extract is not as effective as 0.2% chlorhexidine, whose mean zone of inhibition was 18.36 mm. Less activity of tulsi might be due to less amount of tulsi leaf extract (100 g) used in this study, but even according to a study conducted by Gupta et al. [29] in 2013, 40% concentration of tulsi leaves showed less antimicrobial activity than that of sodium hypochlorite. Eugenol (l-hydroxy-2-methoxy-4-allylbenzene), the active constituent present in Ocimum sanctum, is perhaps largely responsible for the therapeutic potential of tulsi. The other important constituents include ursolic acid and carvacrol. The antimicrobial activity of tulsi can be attributed to these constituents. In comparison with herbal medicines, tulsi is abundantly available, easily accessible, economically feasible, culturally acceptable, and may possess minimal side effects; hence, it can be recommended for long-term use [28].
In this study, aqueous extract of tulsi was used, as it shows better activity against Enterococcus faecalis. But according to Agarwal et al. [8], there was no statistical difference between the efficacies of alcoholic over aqueous extract. An important characteristic of plant extracts and their components is their hydrophobicity, which enables them to partition lipids of the bacterial cell membrane and mitochondria, disturbing the cell structures and rendering them more permeable [14].
In this study, pomegranate extract had a mean zone of inhibition at 100% concentration of 14.50 mm, which was in agreement with that of Subramaniam et al. [30]. This study revealed that pomegranate extract is not as effective as compared to 0.2% chlorhexidine, whose mean zone of inhibition was 18.36 mm. Less activity of pomegranate might be due to less amount of pomegranate fruit used (100 g). Another reason might be that the aqueous extract of pomegranate was used. According to Prashanth et al. [24], the comparison of petroleum ether, chloroform, methanol, and water extracts of Punica granatum, the methanolic extract was found to be most effective against all tested microorganisms.
In our study, pomegranate extract was effective against Enterococcus faecalis at 100% concentration. However, an earlier study found methanolic extract of pomegranate peel to be effective against Streptococcus mutans, Lactobacillus acidophilus, and Streptococcus salivarius only at concentrations of 8 and 12 mg/mL [16]. In vitro studies do not reproduce the exact oral conditions. This was only a preliminary in vitro study that demonstrated the antimicrobial effect of pomegranate on Enterococcus faecalis. Therefore, more clinical trials using different concentrations of Punica granatum Linn extract are necessary to verify its action upon another oral microflora in vivo.
In this study, the least antimicrobial activity among all five medicaments was shown by garlic extract, with a mean zone of inhibition at 100% concentration of 1.40 mm. The least activity of garlic extract might be due to a smaller number of garlic bulbs used (100 g). Another reason might be that the aqueous extract of garlic was used. Therefore, future studies are recommended to investigate the antibacterial effect of garlic extract with a larger amount of garlic bulbs. Also, further studies should be carried out to bring this extract into use in dentistry.
However, there is scarce information on the quality, safety, and greater efficiency of these products for use in dentistry. As most of the studies are carried out ex vivo [31], more of these medicaments should be subjected to animal and human studies to determine their effectiveness, side effects, toxicity, and drug interactions.
Conclusion
The current study suggested that all four herbal products (pomegranate, tulsi, garlic, and liquorice) possess antimicrobial activity against Enterococcus faecalis. Group E (liquorice) extract had maximum antimicrobial activity, followed by Group C (tulsi), Group B (pomegranate), and Group D (garlic) extract, having the least antimicrobial activity. Therefore, it was concluded that it supports the folkloric usage of the herbal extracts (pomegranate, tulsi, garlic, and liquorice) and suggests that these extracts possess antimicrobial properties, but less compared to 0.2% chlorhexidine, to be used as a root canal irrigant. However, further in vivo studies are required to determine the real potential usefulness, side effects, cytotoxicity, and other properties of these plants to be used as effective and safer root canal irrigants.
References
- Eswar K, Venkateshbabu N, Rajeswari K, et al. Dentinal tubule disinfection with 2% chlorhexidine, garlic extract, and calcium hydroxide against Enterococcus faecalis by using real-time polymerase chain reaction: an in vitro study. J Conservat Dentistry. 2013;16(3):194-98.
- Pujar M, Makandar S. Herbal usage in endodontics- A review. Int J Contemp Dentistry. 2011;2(1):34-37.
- Siqueira JF, Rocas IN. Exploiting molecular methods to explore endodontic infections: part 2 – redefining the endodontic microbiota. J Endod. 2005;31(6):488-98.
- Estrela C, Silva JA, Alencar AH, et al. Efficacy of sodium hypochlorite and chlorhexidine against Enterococcus faecalis – a systematic review. J Appl Oral Sci. 2008;16(6):364-8.
- Prasannabalaji N, Muralitharan G, Sivanandan RN, et al. Antibacterial activities of some Indian traditional plant extracts. Asian Pac J Trop Dis. 2012;2(Suppl1):291-5.
- Lansky EP. Beware of pomegranates bearing 40% ellagic acid. J Med Food. 2006;9(1):119-22.
- Shukla A, Kaur K, Ahuja P. Tulsi the medicinal value. Online Int Interdiscip Res J. 2013;3:9-14.
- Agarwal P, Nagesh L. Evaluation of the antimicrobial activity of various concentrations of tulsi (Ocimum sanctum) extract against Streptococcus mutans: an in vitro study. Indian J Dent Res. 2010;21(3):357-9.
- Fujisawa H, Watanabe K, Suma K, et al. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci Biotechnol Biochem. 2009;73(9):1948-55.
- Irani M, Sarmadi M, Bernard F, et al. Leaves antimicrobial activity of Glycyrrhiza glabra L. Iran J Pharm Res. 2010;9(4):425-8.
- Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29(9):565-6.
- Rôças IN, Siqueira JF, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30(5):315-20.
- Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31(1):53-6.
- Aly AM, Al-Alousi L, Salem HA. Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech. 2005;6(1):74-82.
- Bulacio MA, Cangemi R, Cecilia M, et al. In vitro antibacterial effect of different irrigating solutions on Enterococcus faecalis. Acta Odontol Latinoam. 2006;19(2):75-80.
- Wang CS, Arnold RR, Trope M, et al. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endod. 2007;33(11):1283-9.
- Malek JM, Ghazvini K. In vitro susceptibility of Helicobacter pylori to liquorice extract. Iran J Pharm Res. 2007;7(1):69-72.
- Bodet C, La VD, Gafner S, et al. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J Periodontol. 2008;79(9):1752-61.
- Souza-Filho FJ, Soares AD, Vianna ME, et al. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J. 2008;19(1):28-33.
- Meghashri SG. In vitro antifungal and antibacterial activities of root extract of Glycyrrhiza glabra. J Appl Sci Res. 2009;4:1436-9.
- Ballal NV, Kundabala M, Bhat KS, et al. Susceptibility of Candida albicans and Enterococcus faecalis to chitosan, chlorhexidine gluconate, and their combination in vitro. Aust Endod J. 2009;35(1):29-33.
- Badr AE, Omar N, Badria F. A laboratory evaluation of the antibacterial and cytotoxic effect of liquorice when used as a root canal medicament. Int Endod J. 2010;44(1):51-8.
- Sultana S, Haque A, Hamid K, et al. Antimicrobial, cytotoxic and antioxidant activity of methanolic extract of Glycyrrhiza glabra. Agric Biol J N Am. 2010;1(5):957-60.
- Prashanth D, Asha MK, Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001;72:171-3.
- Aberna RA, Prabhakar K. Antienterococcal and betalactam resistance modulating potential of Ocimum sanctum Linn. J Pharm Res. 2011;4:2057-9.
- Mondal S, Varma S, Bamola VD, et al. Double-blinded randomized controlled trial for immunomodulatory effects of tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136(3):452-6.
- Costa EM, Araújo EA, Medeiros AC, et al. In vitro evaluation of the root canal cleaning ability of plant extracts and their antimicrobial action. Braz Oral Res. 2012;26(3):215-21.
- Subbiya A, Mahalakshmi K, Pushpangadan S, et al. Antibacterial efficacy of Mangifera indica L. kernel and Ocimum sanctum L. leaves against Enterococcus faecalis dentinal biofilm. J Conserv Dent. 2013;16(5):454-7.
- Gupta A, Duhan J, Tewari S, et al. Comparative evaluation of antimicrobial efficacy of Syzygium aromaticum, Ocimum sanctum and Cinnamomum zeylanicum plant extracts against Enterococcus faecalis: a preliminary study. Int Endod J. 2013;46(8):775-83.
- Subramaniam P, Dwivedi S, Uma E, et al. Effect of pomegranate and Aloe vera extract on Streptococcus mutans: an in vitro study. Dent Hypotheses. 2012;3:99-105.
- Nagaveni NB, Khan MM, Poornima P. Comparative evaluation of antimicrobial efficacy of chlorhexidine and herbal root canal irrigant Aloe vera against Enterococcus faecalis: an in vitro study. CODS J Dent. 2016;8(2):70-3.
![]() 2 and Afreen Kauser3
2 and Afreen Kauser3



