Essola JK *1,2, Nzinkeu Amougou AL1, Djim-Adjim-Ngana K
*1,2, Nzinkeu Amougou AL1, Djim-Adjim-Ngana K *3,4, Nsa’amang Eyebe C1, Eyebe RH4, Embolo Enyegue EL
*3,4, Nsa’amang Eyebe C1, Eyebe RH4, Embolo Enyegue EL 4, Essomba NE1,2, Guy PN1,5 and Adiogo D1
4, Essomba NE1,2, Guy PN1,5 and Adiogo D1
1Faculty of Medicine and Pharmaceutical Sciences (FMSP), University of Douala, Douala, Cameroon
2Clinical Biology Laboratory, Laquintinie Hospital, Douala, Cameroon
3School of Veterinary Medicine and Sciences, University of Ngaoundere, Ngaoundere, Cameroon
4Centre for Research on Health and Priority Pathologies, Institute of Medical Research and Medicinal Plant Studies, Yaounde, Cameroon
5Gyneco-Obstetric and Pediatric Hospital of Douala, Douala, Cameroon
*Correspondence: Josiane Kikie Essola, Faculty of Medicine and Pharmaceutical Sciences (FMSP), University of Douala, Douala, Cameroon and Clinical Biology Laboratory, Laquintinie Hospital, Douala, Cameroon; Karyom Djim-Adjim-Ngana, School of Veterinary Medicine and Sciences, University of Ngaoundere, Ngaoundere, Cameroon and Centre for Research on Health and Priority Pathologies, Institute of Medical Research and Medicinal Plant Studies, Yaounde, Cameroon
Received on 15 February 2024; Accepted on 04 April 2024; Published on 10 April 2024
Copyright © 2024 Essola JK, et al. This is an open-access article and is distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Platelet antigens called human platelet antigens (HPA) present on the surface of platelets are involved in immunological conflicts, sometimes leading to severe thrombocytopenia related to anti-HPA1a antibodies. The aim of this study was to detect platelet antibodies in newborns and the associated risk factors.
Methods: We conducted a cross-sectional analytical study from 05 January to 30 June 2017 at the Laquintinie Hospital of Douala and the Gyneco-Obstetric and Pediatric Hospital of Douala among newborns with thrombocytopenia and mothers in compliance with ethical considerations. Socio-demographic and clinical data were collected. Blood was collected on EDTA tubes for newborns and on tubes without anticoagulants for mothers in order to determine the presence of anti-HPA1a antibodies. Statistical analysis was performed using SPSS version 22 software.
Results: A total of 35 newborns and 35 mothers were recruited in this study. The mean age of the newborns was 8.5 ± 7.2 days, with a sex ratio of 1.7% in favor of boys. The prevalence of neonatal thrombocytopenia was 4.1%, and the prevalence of HPA1a antibody was 17.1%. Most of the newborns were born to primiparous mothers (57.1%), and 80.0% had prematurity and neonatal jaundice as reasons for hospitalization. Male newborns and those whose mothers had been transfused at least once during or before pregnancy had respectively 4 (OR = 3.53; P-value < 0.0001) and 14 (OR = 14; P-value = 0.0483) times more risk of having the anti-HPA1a antibody.
Conclusion: Our study has shown that the anti-HPA1a antibody is a risk factor for neonatal thrombocytopenia and is associated with maternal transfusion.
Keywords
thrombocytopenia, anti-HPA1a antibody, maternal blood transfusion, Douala
Abbreviations
HPA: human platelet antigens; MAIPA: monoclonal antibody-specific immobilization of platelet antigens; ELISA: enzyme-linked immunosorbent assay; OD: optical density; OR: odds ratio; NAIT: neonatal alloimmune thrombocytopenia
Introduction
Platelet antigens called human platelet antigens (HPA) on the surface of platelets are involved in immunological conflicts such as post-transfusion purpura, platelet transfusion refractory state, and maternal-fetal alloimmunization [1]. The seriousness of this condition lies essentially in the risk of intracranial hemorrhage in 20% of cases, half of which occur in utero; it is the cause of death in 10–15% of cases or of serious neurological sequelae in 15–30% [1]. The incidence of these thrombocytopenias is often estimated at 1 case per 800 to 1000 births, and in 78–90% of cases, the cause is anti-HPA1a antibody [2–4]. Although these thrombocytopenias are rare, the first pregnancy is often affected in 50% of cases, and the presence of anti-HPA1a antibodies increases the risk of recurrence in subsequent pregnancies [2]. Their early detection allows better management of neonatal thrombocytopenia due to alloimmunization. From all the above, very little data are available in the world, in Africa and particularly in Cameroon, on thrombocytopenia related to platelet alloimmunization in newborns due to the absence of systematic screening for these incompatibilities. The aim of this study is to analyze, through a prospective study, the proportion of platelet antibodies to anti-HPA1a in newborns presenting thrombocytopenia in our context.
Materials and Methods
Design of the study
This study was conducted in the city of Douala, located in the coastal region of Cameroon. It was a prospective study conducted from 05 January to 30 June 2017. The study population consisted of mothers and their newborns who were admitted or came to the outpatient clinic for primary hemostasis or hemolysis disorders recruited from the Neonatology and Maternity Departments of the Gyneco-Obstetric and Pediatric Hospital of Douala and the Laquintinie Hospital of Douala. The biological analyses were carried out at the Clinical Biology Laboratory Unit of the Gyneco-Obstetric and Pediatric Hospital of Douala for the analysis of the samples. All mothers whose newborns had thrombocytopenia and all thrombocytopenic newborns whose mothers gave free and informed consent were included, and all false thrombocytopenias were excluded. The sampling size was defined in convenience, and the recruitment procedure was done on a non-probability basis. All those who met the inclusion criteria were recruited. Sample quality was determined by consecutive exhaustive sampling.
Ethical considerations
Prior to the implementation of the study, ethical approvals were obtained from the Institutional Committee of Ethics for Human Health Research of the University of Douala (Ref. CEI-UDo/720/01/2017/M). Finally, a research authorization was obtained from the officials of the Gyneco-Obstetric and Pediatric Hospital of Douala and the Laquintinie Hospital of Douala.
Laboratory analysis
After obtaining free and informed consent, the patients (mothers) completed a questionnaire based on socio-demographic characteristics (age, sex, and birth weight for newborns) (age, marital status, level of education, and occupation for mothers) and clinical and biological characteristics (bleeding, purpura, history of thrombocytopenia). Then, two samples were taken from the elbow.
- One in a 2 ml pediatric tube with EDTA anticoagulant for newborns to screen for anti-HPA1a antibodies
- The other in a dry tube without anticoagulants for the mother to identify the anti-HPA1a antibody
- Analytical phase: this was done in three steps
Blood count: This count was carried out on the Sysmex XN 1000 (automatic blood count machine), which uses the principle of flow cytometry and enabled us to identify RBCs, WBCs, and platelets, to measure BH, to calculate and measure the hematocrit, the VGM, and the erythrocyte constants, and to establish an approximate leucocyte formula. The parameter that interested us in this case was the platelet count, which had to be less than 150 G/L.
The making of the blood smear: Carried out on a slide and stained with May-Grunwald-Giemsa (MGG) stain, it is the cytological study of the figurative elements of blood. In our study, we checked for the presence of platelet aggregates and giant platelets in order to confirm thrombocytopenia.
Detection of HPA1a antibody: Detection of HPA1a antibody was performed using the Monoclonal Antibody-Specific Immobilization of Platelet Antigens (MAIPA) technique, which is a qualitative test used to screen for antiplatelet antibodies in serum or plasma (MAIPA indirect) and/or to detect antibodies bound to a patient’s platelets (MAIPA direct). A positive MAIPA indirect or MAIPA direct test result requires subsequent identification of antibody specificity using the same method.
The principle of the test is based on the capture of a platelet antigen using mouse monoclonal antibodies to human platelet membrane glycoproteins and the analysis of the binding of human antibodies to this antigen by an enzyme-linked immunosorbent assay (ELISA).
Throughout our study, we performed the direct MAIPA test in newborns with a platelet count of less than 150 G/L with the absence of platelet aggregates on the smear slide in order to detect platelet-bound antibodies in the latter. Antibody identification was done with sera from mothers with positive newborn screening.
Reading and interpretation of results
- Validation and interpretation of screening tests
The validation of the screening test depended on the optical density (OD) values of the control samples. After subtraction of the blank OD value:
- The OD value for the negative control had to be less than 0.1
- The OD value for the positive control should be greater than 0.5
Interpretation of the tests is relatively simple,
With an OD cut-off value of 0.2 (after subtraction of the blank value):
- OD values above 0.2 were considered positive
- OD values below 0.2 were considered negative
All positive screening test results were confirmed by identification testing in the mothers of each corresponding newborn.
Statistical analysis
The data were entered into an Excel sheet (Microsoft Office 2007) and subsequently analyzed with SPSS 22 for Windows (SPSS, Inc., Chicago, IL, USA). The variables were presented as percentages (95% confidence interval) in tables and graphs (pie charts and histograms). Fisher’s exact test (bivariate statistics) and Chi-square test of homogeneity (univariate statistics) were used to compare proportions. Logistic regression (univariate model) was used to identify factors associated with the presence of HPA1a antibody. This test was used to quantify the association between the dependent variable (presence of HPA1a antibody) and the independent variables through the calculation of the odds ratio (OR) and its 95% confidence interval. The significance threshold was set at a P-value < 0.05.
Results
Socio-demographic, clinical, and biological characteristics of the study population
During this study, we registered 1134 newborns, of whom 855 had a blood count, and only 35, after elimination of false thrombocytopenia, were retained with the 35 corresponding mothers. The most represented age range of newborns was between 0 and 7 days (51.4%), as shown in the figure (Figure 1). The average age of the babies was 8.5 ± 7.2 days, with extreme values of 1 and 28 days (Figure 1).
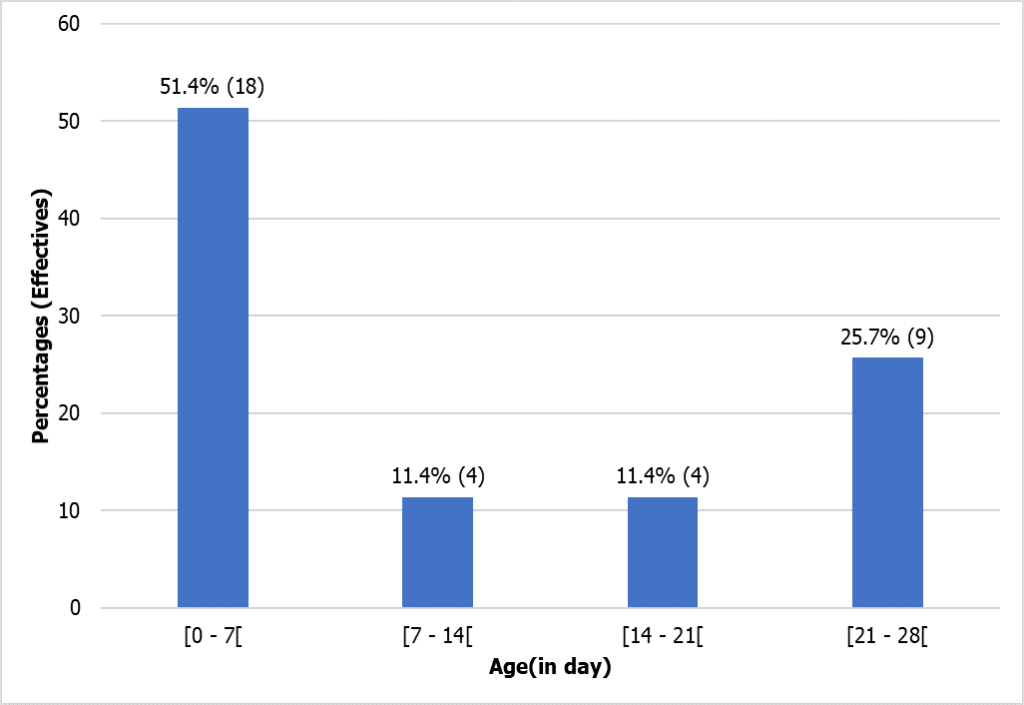
Figure 1: Chronological age distribution of newborns.
Most of the newborns were not born at full term, 25 beyond 37 weeks of amenorrhea, corresponding to 71.4% of the study population, very premature babies, and medium premature babies, representing 28.6% of the total study population. The average gestational age was 34.1 ± 3.9 weeks of amenorrhea, with extreme values of 28 and 41 weeks.
In this study, most newborns had a weight of less than 2500 g and were from mono-fetal pregnancies, as shown in the table (Table 1).
| | Single foetal | Twinned |
| Birth weight | Workforce | Female | Male | Female | Male |
| < 2500 g | 28 | 9 | 14 | 2 | 3 |
| 2500–4000 g | 7 | 2 | 4 | 0 | 1 |
| > 4000 g | 0 | 0 | 0 | 0 | 0 |
| Total | 35 | 11 | 18 | 2 | 4 |
Table 1: Birth weight by gender and pregnancy type.
The majority of thrombocytopenias found during our study were moderate thrombocytopenia represented here at 40.0%, and very few have very severe thrombocytopenia (less than 20,000 G/L), as shown in the figure (Figure 2).
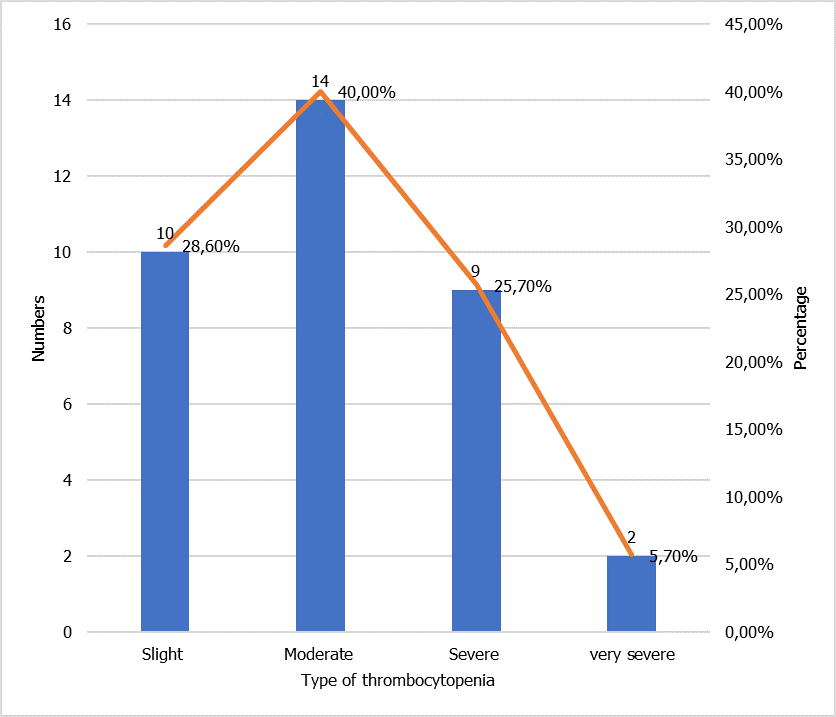
Figure 2: Distribution according to the severity of thrombocytopenia.
Prevalence of HPA1a antibody
Of the 35 newborns with thrombocytopenia, 6 tested positive for the anti-HPA1a antibody, giving a prevalence of 6/35 or 17.1%.
Risk factors associated with HPA1a
The table (Table 2) shows that male and transfused babies were almost 4 times (OR = 3.53; P-value < 0.0001) and 14 times (OR = 14; P-value = 0.0483) more likely to be HPA1a positive compared to female and non-transfused babies respectively.
| Variables | Modalities | Effective | HPA1 (+) | OR (IC95%) | P-value |
| Gender | Female | 13 | 1 (7,7%) | 1 | |
| Male | 22 | 5 (22,7%) | 3,53 (1,36 – 34,19) | < 0,0001 |
| Age of babies (days) | [0–7] | 18 | 3 (16,7%) | 1 | |
| [7–14] | 4 | 0 (0,0%) | 2,50 (0,00 – 4,20) | 0,9749 |
| [14–21] | 4 | 2 (50,0%) | 5,00 (0,49 – 50,84) | 0,1738 |
| [21–28] | 9 | 1 (11,1%) | 0,63 (0,06 – 7,03) | 0,7035 |
| Blood transfusion | No | 32 | 4 (12,5%) | 1 | |
| Yes | 3 | 2 (66,7%) | 14,0 (1,02 – 192,18) | 0,0483 |
| Blood type | A | 10 | 1 (10,0%) | 1 | |
| B | 8 | 1 (12,5%) | 1,29 (0,07 – 24,39) | 0,8671 |
| O | 16 | 4 (25,0%) | 3,0 (0,28 – 31,64) | 0,3607 |
| Gestational age | Premature | 10 | 2 (20,0%) | 1 | |
| Average | 15 | 3 (20,0%) | 1,0 (0,14 – 7,39) | 1,0000 |
| Born at term | 10 | 1 (10,0%) | 0,44 (0,03 – 5,88) | 0,5383 |
| Type of thrombocytopenia | Slight | 10 | 2 (20,0%) | 1 | |
| Moderate | 14 | 1 (7,1%) | 0,31 (0,02 – 3,96) | 0,3663 |
| Severe | 9 | 3 (33,3%) | 2,0 (0,25 – 15,99) | 0,5134 |
Table 2: Factors associated with HPA1a antibody. OR = odds ratio; CI 95% = 95% confidence interval; P-value < 0.05 were considered significant; OR < 1 (protective factor); OR = 1 (neutral factor); OR > 1 (risk factor).
Discussion
Our study focused on the role of the anti-HPA1a antibody in neonatal thrombocytopenia at the Laquintinie Hospital and the Gyneco-Obstetric and Pediatric Hospital of Douala. 1,134 newborns were registered during the study, of which 855 had a blood count, and only 35 were diagnosed to have thrombocytopenia, representing 4.1%. The most represented age group was between 0 and 7 days, 51.4% of cases, early thrombocytopenia predominated. 22 male newborns, 62.9%, and 13 female newborns, 37.1%, with a sex ratio of 1.7; these results are comparable to those reported in 2010 in Tunisia by Guerinik [5] who working on neonatal thrombocytopenia found the same age group at 88.82%, the predominance of male represented at 61% against 39% for the female. The majority of the newborns were premature (71.4%), of which 28.6% were very premature and 42.8% medium premature. Moreover, 80.0% of the babies had a birth weight of less than 2500 g. These results differ from those obtained in Cameroon in 2007, which was 77.38% of newborns at term with a weight between 2500 g and 4000 g [6]. This difference could be related to the maternal history or to the fact that the newborns in our study were all thrombocytopenic at the base.
The prevalence of neonatal thrombocytopenia obtained in our study was 4.1%, with 5.7% of cases of very severe thrombocytopenia, 25.7% of severe thrombocytopenia, 40.0% of moderate thrombocytopenia, and 28.6% of mild thrombocytopenia. These data are different from those obtained in Morocco in 2008, i.e., 2.00% and 23.3%, respectively. This discrepancy could be related to the variability of the study environment.
The majority of newborns were born in primiparous pregnancies at 51.4% with a significant difference and a p-value of less than 0.0001. This result is different from that observed in Benin in 2005 by Anani et al. [7], who found 24.8%. Neonatal jaundice and prematurity were the main reasons for hospitalization at 80.0% and 50.0%, respectively. Prematurity and neonatal jaundice could, therefore, be factors associated with neonatal thrombocytopenia and could be explained, as stated in the literature, by the immaturity of platelet function and hepatitis during the neonatal period.
Maternal-fetal incompatibility in the platelet system (HPA) can lead to neonatal alloimmune thrombocytopenia (NAIT). Thus, we obtained 17.14% of anti-HPA1a antibodies in our study population. These antibodies were systematically found in the mothers of children with alloantibodies. Ouabdelmoumene et al. [9] found a risk frequency of alloimmunization of mothers of 20.63% against the HPA1a antigen; however, different and variable data were reported with frequencies of 95%, 10.6%, and 79.2% of mothers with anti-HPA1a alloantibodies, respectively [2, 7–9]. This discrepancy could be explained by the variability and diversity of the population, the size of the population, and the duration of the study. However, in Caucasians, the HPA-1 system is most involved in NAIT, while in Japanese, HPA-2b is responsible for ineffective platelet transfusions, and HPA-4b is involved in NAIT. The main risk factors associated with the presence of the antibody were gender and blood transfusion. Our study showed that male newborns and those whose mothers had been transfused were almost 4 times (OR = 3.53; P-value < 0.0001) and 14 times (OR = 14; P-value = 0.0483) more likely to be HPA1a antibody positive compared to female newborns and those whose mothers had not been transfused. This could be explained by the fact that our study population was predominantly primiparous and had received blood transfusions and that transfusion compatibility is often limited in Africa and does not involve testing for platelet markers. In our study, 3 of the newborns who tested positive for HPA1a antibody were from mothers who had been transfused at least once during or before pregnancy and would probably have become immune to the platelet allo antigens they had received from the various transfusions.
Conclusion
The frequency of neonatal thrombocytopenia was 4.1%, the presence of anti-HPA1a antibodies in 17.1% of thrombocytopenic subjects, the predominance of males among thrombocytopenic subjects with anti-HPA1a antibodies, the role of blood transfusion in the development of anti-HPA1a antibodies, and the fact that anti-HPA1a antibodies play a significant role in neonatal thrombocytopenia so that immunological neonatal thrombocytopenia is also a reality in our working environment. We need to take up the assessment of the state of the art of anti-platelet immunization in a more comprehensive way.
Ethics Approval and Consent to Participate
The National Ethics Committee for Human Health Research (n° 2020/18/082/CE/CNERSH/SP) approved the experimental protocols and methods used in this study. The study was performed for the revised Helsinki Declaration (1989). All methods were carried out according to relevant guidelines and regulations. Informed consent was obtained from all individual participants included in the study. The study objectives were explained to each participant and their parents/guardians, and written informed consent was obtained prior to their inclusion in the study.
What is Known About this Topic?
- The incidence of these thrombocytopenias is often estimated at 1 case per 800 to 1000 births, and in 78–90% of cases, the cause is anti-HPA1a antibody.
- Platelet antigens called HPA are involved in immune conflicts that can lead to severe thrombopenia linked to the anti-HPA1a antibody.
What this Study Adds
- This study found a 4.1% incidence of neonatal thrombocytopenia.
- Anti-HPA1a antibodies were present in 17.1% of patients with thrombopenia.
- The newborns were mostly from primary mothers at 57.1% and had premature pregnancy and neonatal icter at 80.0% for hospitalization reasons.
Author Contributions
All authors contributed to the design and execution of the study, participated in article drafting and critical revision, and read and approved the final version of the manuscript.
Funding
This study did not receive any funding in any form.
Availability of Data and Materials
The data will be available upon reasonable request to the corresponding author.
Competing Interests
The authors declare no competing interests.
Acknowledgments
The authors thank all the women who agreed to participate in the study with their children. They also express their gratitude to all the administrative and medical staff of the relevant structures that facilitate the implementation of this study at each study site.
References
- Bertrand G, Kaplan C. Génotypage en immunologie plaquettaire : quand ? Comment ? Limites. Transfus Clin Biol. 2009;16(2):164-69.
- Toughza J, Agadr A, Nejjari M, et al. Diagnostic et prise en charge d´une thrombopénie néonatale sévère par allo-immunisation materno-fœtale: rapport d´un cas et revue de la littérature [Diagnosis and management of severe neonatal thrombocytopenia due to maternal alloimmunization against fetal platelet antigens: case study and literature review]. Pan Afr Med J. 2020;(37):382.
- Martageix C, Bianchi F, Casale C, et al. Allo-immunisation plaquettaire et manifestations cliniques dans le contexte maternofœtal. Transfus Clin Biol. 2019;26(3):15.
- Bertrand G, Drame M, Martageix C, et al. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood. 2011;117(11):3209-213.
- Guerinik MEA. Thrombopénies néonatales. 2010.
- Memoire Online. Les infections néonatales bactériennes dans l’unité de néonatalogie de l’hôpital gynéco-obstétrique et pédiatrique de Yaoundé. Biologie et Médecine. 2007.
- Anani L, Bigot A, Latoundji S, et al. L’incompatiblite foeto-maternelle anti-plaquettaire: a propos de 238 gestantes a Cotonou. J Société Biol Clin Bénin. 2005;10–15.
- Brojer E, Husebekk A, Dębska M, et al . Fetal/Neonatal Alloimmune Thrombocytopenia: Pathogenesis, Diagnostics and Prevention. Arch Immunol Ther Exp (Warsz). 2016;64(4):279-90.
- Ouabdelmoumene Z, Housse El H, Zarati F, et al. Polymorphisme des antigènes plaquettaires humains HPA chez les donneurs de sang au Maroc. Transfus Clin Biol. 2016;23(4):296.
![]() *1,2, Nzinkeu Amougou AL1, Djim-Adjim-Ngana K
*1,2, Nzinkeu Amougou AL1, Djim-Adjim-Ngana K![]() *3,4, Nsa’amang Eyebe C1, Eyebe RH4, Embolo Enyegue EL
*3,4, Nsa’amang Eyebe C1, Eyebe RH4, Embolo Enyegue EL![]() 4, Essomba NE1,2, Guy PN1,5 and Adiogo D1
4, Essomba NE1,2, Guy PN1,5 and Adiogo D1

