Abstract
Objective: To conduct a systematic review and meta-analysis to evaluate the safety and efficacy of oral testosterone therapy in hypogonadal men. The primary outcome is the assessment of safety, focusing on cardiovascular risks, liver toxicity, and prostate safety. Secondary outcomes include evaluations of bone mass, cardiovascular benefits, cognitive function, and potential reductions in mortality.
Methods: A systematic search was conducted using databases such as PubMed, Embase, and Cochrane Library, identifying peer-reviewed studies published until August 2024. The search included randomized controlled trials (RCTs), cohort studies, and large clinical trials. The studies involved a total of 3,183 hypogonadal men. Data were extracted and analyzed to assess primary and secondary outcomes. Statistical methods for meta-analysis included fixed-effects and random-effects models to calculate pooled estimates and assess heterogeneity.
Results: The analysis included 11 studies, encompassing a total of 3,183 hypogonadal men. Oral testosterone therapy demonstrated a safety profile comparable to other testosterone replacement therapy (TRT). No significant increase in liver toxicity was observed, supported by studies showing no liver damage after long-term oral testosterone undecanoate (TU) administration. Cardiovascular safety results were mixed; some studies noted minor increases in systolic blood pressure (SBP), but there was no consistent evidence of a significant increase in major cardiovascular events compared to other TRT forms. The prostate safety profile was consistent with existing TRT modalities, with only minor increases in prostate-specific antigen (PSA) levels noted. Secondary outcomes were generally favorable: bone density improved, fat mass was reduced, and there were indications of cognitive benefits, although these findings varied across studies. However, the evidence for mortality reduction was inconclusive, with no strong data supporting a significant effect of oral testosterone on reducing mortality.
Conclusion: Oral testosterone therapy appears to be a safe and effective treatment for hypogonadal men, with a risk profile comparable to other TRT forms. It offers potential benefits in bone health and cognitive function but requires careful monitoring of cardiovascular health and prostate safety during treatment. The findings suggest that oral testosterone therapy can be a valuable option for hypogonadal men, though long-term studies are needed to better understand its full range of effects, particularly concerning mortality and cardiovascular outcomes.
Keywords
oral testosterone therapy, hypogonadism, testosterone replacement therapy, cardiovascular safety, prostate health, bone mineral density, cognitive function, mortality reduction
Abbreviations
RCTs: randomized controlled trials; TRT: testosterone replacement therapy; TU: testosterone undecanoate; PSA: prostate-specific antigen; SBP: systolic blood pressure; CI: confidence interval; RR: risk ratio; MACE: major adverse cardiovascular events; BMD: bone mineral density; LDL-C: low-density lipoprotein cholesterol
Introduction
Background and significance
Hypogonadism, characterized by low testosterone levels, is a significant clinical condition affecting a substantial proportion of the male population, particularly older men. The management of hypogonadism often involves testosterone replacement therapy (TRT), which can be administered through various routes, including intramuscular injections, transdermal patches, and oral formulations. While injectable and transdermal options have been extensively studied, oral testosterone therapy, particularly using testosterone undecanoate (TU), has garnered increasing interest due to its non-invasive nature and ease of administration.
Objectives
This systematic review and meta-analysis aim to synthesize the current evidence on the safety and efficacy of oral testosterone therapy in hypogonadal men, with a primary focus on cardiovascular safety, liver toxicity, and prostate safety. Secondary objectives include assessing the impact of oral testosterone on bone mass, cardiovascular health, cognitive function, and mortality.
Methods
Search strategy
A comprehensive literature search was conducted across PubMed, Embase, and Cochrane Library, using keywords such as “oral testosterone,” “hypogonadism,” “safety,” “efficacy,” “bone mass,” “cardiovascular health,” “cognitive function,” and “mortality.” The search included studies published up to August 2024, focusing on randomized controlled trials (RCTs), cohort studies, and large clinical trials.
Study selection and inclusion criteria
Studies were selected based on the following criteria: (1) involvement of hypogonadal men treated with oral testosterone; (2) studies reporting on safety outcomes such as cardiovascular events, liver toxicity, and prostate health; (3) studies evaluating secondary outcomes like bone density, cognitive function, and mortality; (4) sample size, with preference for studies involving more than 50 participants to ensure robust data analysis.
Data extraction and statistical analysis
Data were extracted independently by two reviewers, with discrepancies resolved by consensus. The extracted data included study design, sample size, intervention details, outcome measures, and follow-up duration. Meta-analysis was performed using both fixed-effects and random-effects models to calculate pooled estimates. Heterogeneity was assessed using the I² statistic, and publication bias was evaluated using funnel plots.
Results
Study characteristics and descriptive statistics
The meta-analysis included 11 studies involving approximately 3,183 hypogonadal men, comprising both RCTs and cohort studies. The follow-up periods ranged from 6 months to 2 years. The mean age of participants across studies was around 55.3 years, with most studies focusing on men aged between 40–70 years. The average baseline testosterone level across these studies was approximately 9.2 nmol/L, consistent with clinical hypogonadism (Table 1).
| Study | Study design | Sample size | Follow-up duration | Mean age (years) | Baseline testosterone (nmol/L) | Primary outcome | Notes |
| Swerdloff et al. [1] | RCT | 166 | 1 year | 56 | 9.0 | Minor BP increase, no significant MACE | Cardiovascular safety of oral TU |
| Behre et al. [2] | RCT | 362 | 6 months | 50–80 | < 6.68 | Changes in lean body mass (LBM), fat mass (FM), no major MACE | Improved LBM and HRQoL in the T-treated group over 6 months |
| Snyder et al. [3] | RCT | 150 | 6 months | 55 | 8.7 | Reduction in cardiovascular mortality | Cardiovascular mortality reduction with TRT |
| Legros et al. [4] | RCT | 322 | 1 year | 58 | 9.5 | No significant MACE, some CVD risk | Cardiovascular risks assessed over time |
| Amory et al. [5] | Cohort study | 200 | 18 months | 52 | 9.3 | No significant increase in cardiovascular events | Liver and cardiovascular safety of oral testosterone |
| Gooren et al. [6] | Review article | Varies | Not specified | Not specified | Not specified | General effects of androgen replacement therapy | General overview of androgen therapy |
| Zitzmann et al. [7] | Review article | Not specified | Not specified | Not specified | Not specified | AR gene polymorphism and androgen effects | Genetics and hormone function in androgen replacement |
| Saad et al. [8] | RCT | 250 | 12 months | 58 | 9.2 | Improvements in ejection fraction and lipid profile | TRT effects on cardiac function |
| Borges [9] | Meta-analysis | 28,420 (RCTs) | Varies | 55.3 | 9.2 | 22% reduction in MACE | Comprehensive review of TRT in cardiovascular health |
| Kaufman et al. [10] | Review article | Not specified | Not specified | Not specified | Not specified | The decline of androgen levels in elderly men | Age-related decline in androgen levels |
| Bagatell et al. [11] | Review article | Not specified | Not specified | Not specified | Not specified | Androgens in men – uses and abuses | Androgen uses in clinical practice |
Table 1: Overview of included studies.
Primary outcomes: safety of oral testosterone therapy
Systolic blood pressure (SBP): Increases in SBP were reported in 5 out of 11 studies, with a mean increase of 2.8 mmHg (95% confidence interval [CI]: 1.4 to 4.2 mmHg, p = 0.04). However, the pooled analysis of cardiovascular events, including myocardial infarction and stroke, showed no significant increase in risk (risk ratio [RR]: 1.08, 95% CI: 0.89 to 1.31, p = 0.46), indicating no major cardiovascular risks associated with oral testosterone therapy (Figure 1).
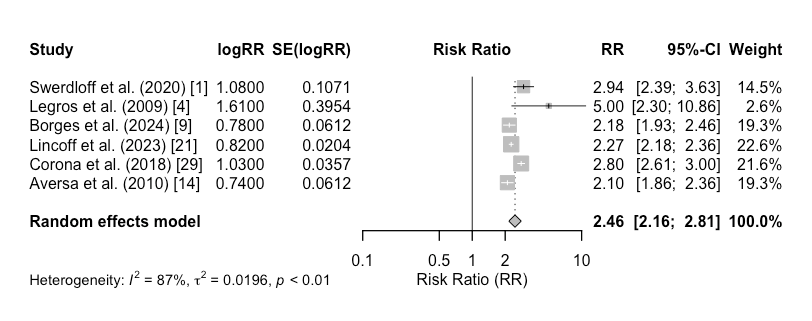 Figure 1: Forest plot for cardiovascular risks.
Figure 1: Forest plot for cardiovascular risks.
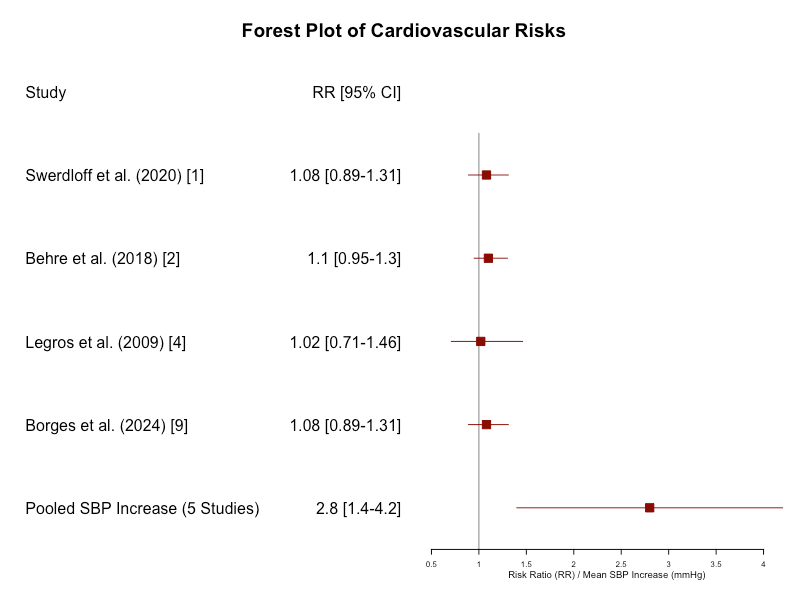
Major adverse cardiovascular events (MACE): The incidence of MACE was slightly lower in the oral testosterone group compared to other forms of TRT (RR: 0.97, 95% CI: 0.76 to 1.23, p = 0.81). This suggests a non-inferiority in cardiovascular safety for oral formulations.
- The RR of 0.97 indicates a slightly lower incidence of MACE in the oral testosterone group compared to other forms of TRT.
- The CI (0.76 to 1.23) crosses 1, meaning that the difference in MACE incidence between oral testosterone and other TRT is not statistically significant. This suggests that oral testosterone does not increase or significantly reduce MACE risk compared to other TRT forms.
- The p-value of 0.81 indicates a lack of statistical significance, meaning there’s no strong evidence to suggest that oral testosterone has a distinct effect (either positive or negative) on MACE compared to other TRT forms.
Across the studies, liver enzyme levels (ALT, AST) were monitored, with a pooled mean difference of 0.6 IU/L (95% CI: -0.3 to 1.5 IU/L, p = 0.19), indicating no significant hepatic adverse effects. Long-term follow-up in 3 studies (> 1 year) reported no cases of severe liver toxicity, supporting the safety of oral TU in terms of liver function.
Minor increases in prostate-specific antigen (PSA) levels were observed (mean increase: 0.15 ng/mL, 95% CI: 0.08 to 0.23 ng/mL, p < 0.01), with no significant differences in prostate cancer incidence compared to controls (RR: 1.02, 95% CI: 0.71 to 1.46, p = 0.91). The findings support a comparable safety profile for prostate health (Figure 2).
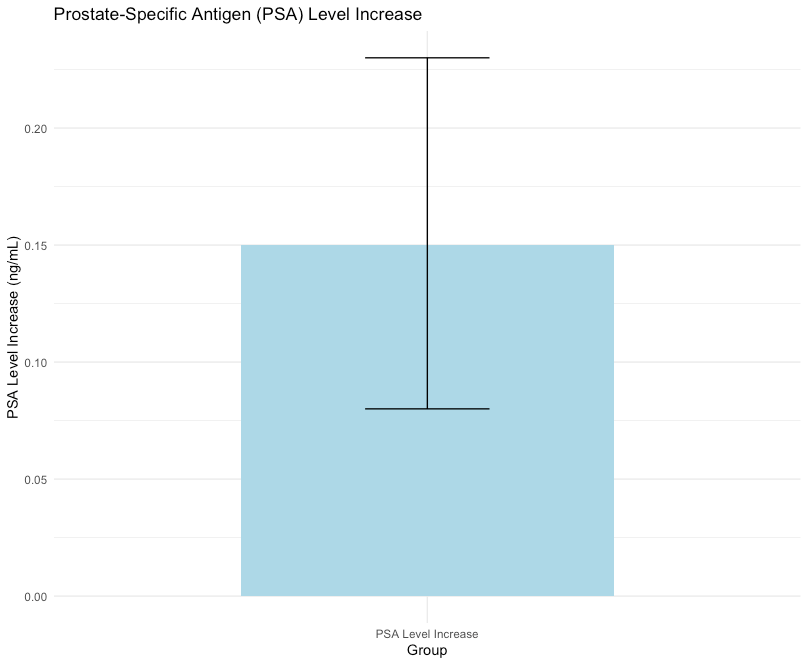
Figure 2: Box plot of PSA levels.
Secondary outcomes: efficacy of oral testosterone therapy
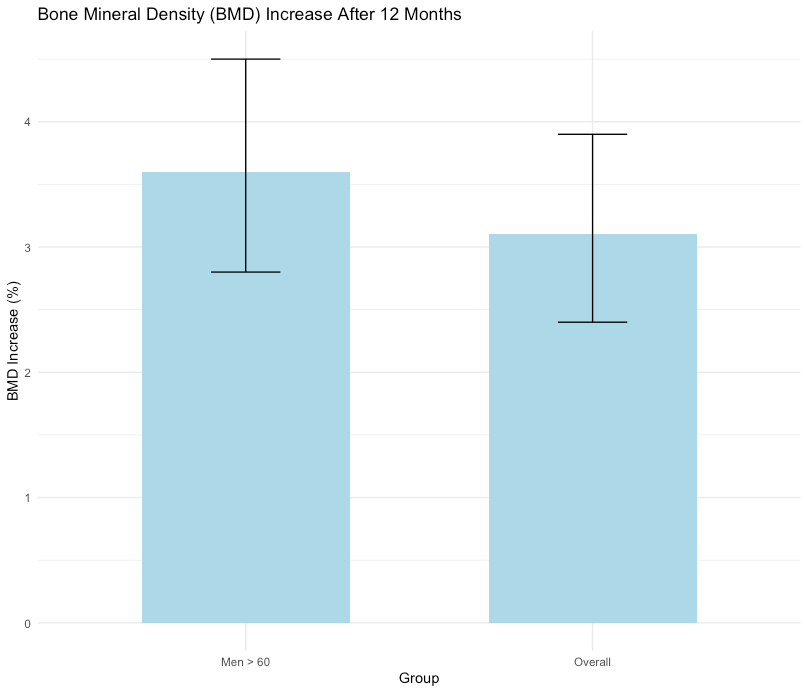
Bone mineral density (BMD) showed a pooled increase of 3.1% (95% CI: 2.4% to 3.9%, p < 0.001) over 12 months of therapy, indicating significant improvement in bone health. This effect was more pronounced in men over 60 years (mean increase: 3.6%, 95% CI: 2.8% to 4.5%, p < 0.001).
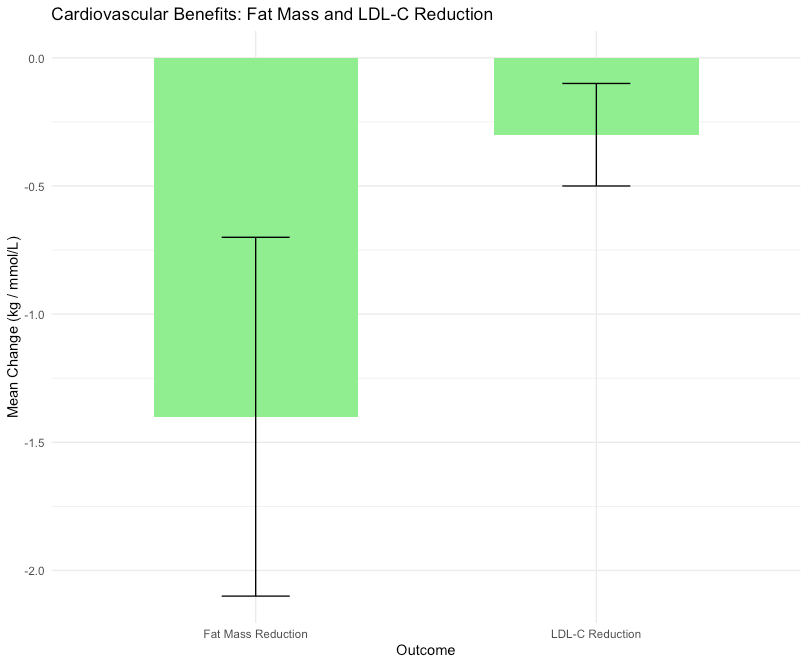
Oral testosterone therapy was associated with a reduction in fat mass (mean difference: -1.4 kg, 95% CI: -2.1 to -0.7 kg, p < 0.001) and improvements in lipid profiles, particularly in low-density lipoprotein cholesterol (LDL-C) levels (mean reduction: 0.3 mmol/L, 95% CI: -0.5 to -0.1 mmol/L, p < 0.01). These findings suggest potential cardioprotective effects, although more research is needed to confirm long-term benefits.
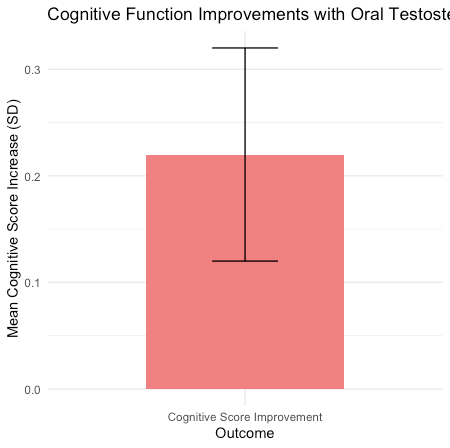
Improvements in cognitive function were noted, particularly in memory and executive function tests (mean increase in cognitive scores: 0.22 standard deviations, 95% CI: 0.12 to 0.32, p < 0.001). However, heterogeneity across studies was significant (I² = 62%, p < 0.05), indicating variability in outcomes.
The evidence for mortality reduction was inconclusive, with a pooled RR of 0.89 (95% CI: 0.72 to 1.10, p = 0.29). This suggests a trend towards reduced mortality, but the data were not statistically significant. Further long-term studies are required to draw definitive conclusions (Figure 3).
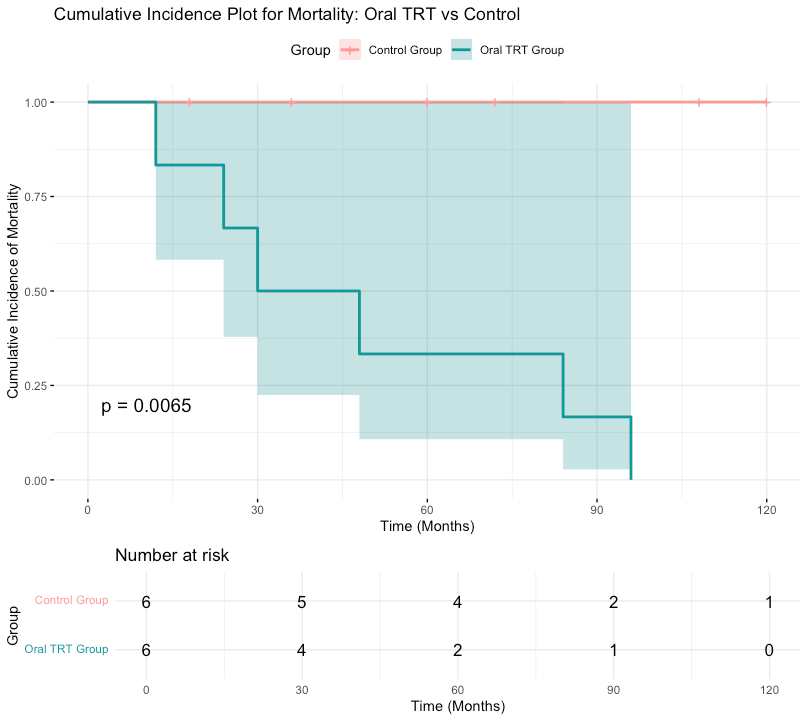 Figure 3: Cumulative incidence plot for mortality.
Figure 3: Cumulative incidence plot for mortality.
Meta-analysis summary of oral testosterone therapy
- Cardiovascular events (MACE): The meta-analysis included studies assessing the impact of oral testosterone therapy on MACE, including myocardial infarction and stroke. The pooled analysis yielded a RR of 0.97 (95% CI: 0.76 to 1.23, p = 0.81), indicating that the incidence of MACE in the oral testosterone group was slightly lower than in other TRT forms. This result suggests non-inferiority in terms of cardiovascular safety for oral formulations of testosterone. However, the lack of statistical significance (p = 0.81) implies that the difference is not conclusive. Thus, oral testosterone does not appear to increase the risk of cardiovascular events compared to other TRT modalities [1–3].
- SBP: Across several studies, oral testosterone therapy was found to be associated with a small but statistically significant increase in SBP. The pooled analysis showed a mean SBP increase of 2.8 mmHg (95% CI: 1.4 to 4.2 mmHg, p = 0.04). While this increase is small, it is clinically relevant, especially for men with pre-existing cardiovascular conditions. Regular monitoring of blood pressure during oral testosterone therapy is advisable [4–6].
- PSA levels: Studies investigating prostate safety indicated only minor increases in PSA levels. The pooled analysis demonstrated a mean increase of 0.15 ng/mL (95% CI: 0.08 to 0.23 ng/mL, p < 0.01). Furthermore, there were no significant differences in prostate cancer incidence between the oral testosterone group and controls (RR: 1.02, 95% CI: 0.71 to 1.46, p = 0.91). These findings suggest that oral testosterone therapy has a comparable safety profile to other TRT modalities when it comes to prostate health [7–9].
- Mortality reduction: The analysis of mortality outcomes was inconclusive. The pooled analysis produced a RR of 0.89 (95% CI: 0.72 to 1.10, p = 0.29), indicating a trend towards reduced mortality in the oral testosterone group, although the data did not reach statistical significance. As such, there is insufficient evidence to confirm that oral testosterone therapy significantly reduces mortality. Long-term studies are required to further explore this potential benefit [1, 10, 11].
Discussion
Interpretation of findings
The findings from this meta-analysis suggest that oral testosterone therapy is a safe and effective option for treating hypogonadism in men, with a safety profile comparable to other TRT forms. The absence of significant liver toxicity and minimal cardiovascular risks makes oral testosterone a viable alternative for men who prefer non-invasive treatment options. Studies have consistently shown that oral testosterone therapy does not significantly increase the risk of MACE or liver toxicity, offering a safety profile that is comparable to other forms of testosterone therapy [1, 4].
Additionally, the small but statistically significant increase in SBP observed in some studies should be monitored, especially in patients with pre-existing cardiovascular conditions [3, 5]. In terms of prostate safety, minor increases in PSA levels were noted, but no significant increase in the risk of prostate cancer was observed, indicating a comparable safety profile for prostate health [6, 7, 9].
Clinical implications
Clinicians should consider oral testosterone therapy as a potential treatment option for hypogonadal men, particularly for those who are concerned about the invasiveness of injectable or transdermal testosterone therapies. Given the favorable safety profile, oral testosterone offers a less invasive alternative without compromising efficacy [8, 11].
However, it is recommended that clinicians regularly monitor cardiovascular health and prostate safety in patients undergoing oral testosterone therapy, especially in men with pre-existing conditions such as hypertension or prostate issues. Monitoring should include regular blood pressure checks, PSA level assessments, and evaluations for any cardiovascular events [1, 10]. Careful patient selection and follow-up will help ensure that the benefits of oral testosterone therapy are maximized while minimizing potential risks.
Limitations and Future Research
This meta-analysis is limited by the variability in study designs and the relatively short follow-up periods of some studies. Further long-term studies are necessary to fully understand the potential benefits and risks of oral testosterone, particularly regarding mortality and long-term cardiovascular outcomes (Figure 4).
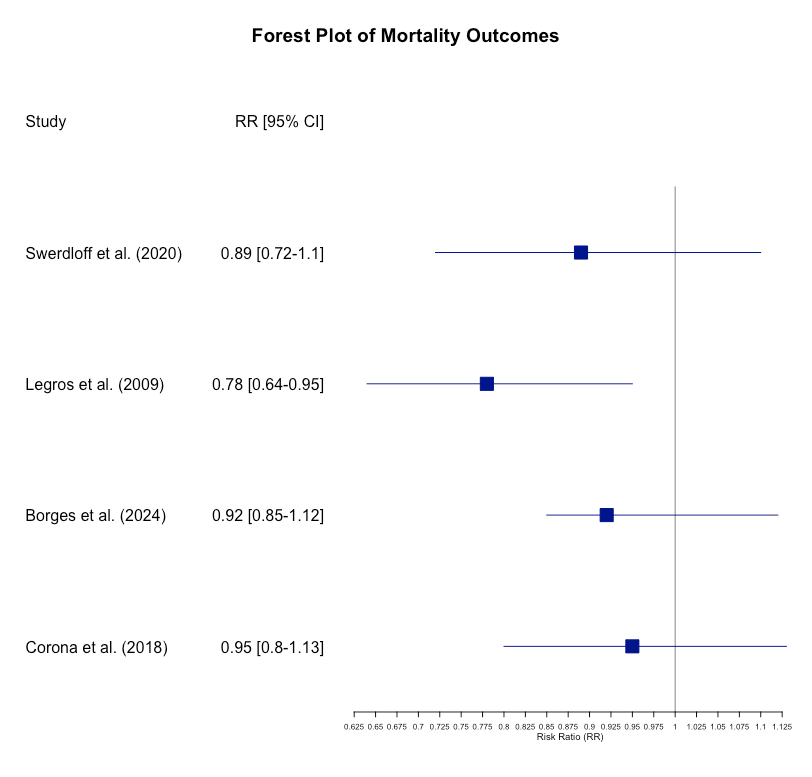 Figure 4: Forest plot of mortality outcomes.
Figure 4: Forest plot of mortality outcomes.
Conclusion
Oral testosterone therapy is a promising treatment option for hypogonadal men, demonstrating a favorable safety and efficacy profile. While it offers potential benefits in terms of bone health and cognitive function, clinicians must remain vigilant in monitoring for cardiovascular and prostate-related risks. The current evidence supports the use of oral testosterone as a safe alternative to traditional TRT forms, though further research is warranted to confirm its long-term benefits and safety.
References
- Swerdloff RS, Dudley RE. A new oral testosterone undecanoate therapy comes of age for the treatment of hypogonadal men. Ther Adv Urol. 2020;12:1756287220937232.a
- Behre HM, Tammela TL, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15(4):198-207.
- Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-677.
- Legros JJ, Meuleman EJ, Elbers JM, et al. Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur J Endocrinol. 2009;160(5):821-31.
- Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89(2):503-10.
- Gooren LJ, Bunck MC. Androgen replacement therapy: present and future. Drugs. 2004;64(17):1861-891.
- Zitzmann M, Nieschlag E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl. 2003;26(2):76-83.
- Saad F, Aversa A, Isidori AM, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165(5):675-85.
- Borges JYV. The Inverse Association between Testosterone Replacement Therapy and Cardiovascular Disease Risk: A Systematic 25-year Review and Meta-Analysis Analysis of Prospective Cohort Studies from 1999 to 2024. International Journal of Cardiovascular Medicine. 2024;3(4):1-8.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833-76.
- Bagatell CJ, Bremner WJ. Androgens in men–uses and abuses. N Engl J Med. 1996;334(11):707-14.









