Obesity and heart failure (HF) prevalence have increased globally; current HF prevalence is estimated to be 64.34 million cases, accounting for 9.91 million years lost due to disability [1, 2], worldwide, 64.3 million people are estimated to suffer from HF in 2017, making it a global pandemic [3]. The highest prevalence rates of HF were observed in Central Europe, North Africa, and the Middle East in 2017, whereas the lowest prevalence rates were observed in Eastern Europe and Southeast Asia [4].
According to World Health Organization (WHO), the worldwide prevalence of obesity nearly tripled between 1975 and 2016. Overall, about 13% of the world’s adult population (11% of men and 15% of women) were obese in 2016. Overweight and obese children under the age of five accounted for 38.2 million of all children in 2019 [5]. In Saudi Arabia, the rate of overweight and obesity increased dramatically among citizens, in contrast to worldwide projections (35% vs. 13%) [6]; obesity prevalence increased from 22% in 1990–1993 to 36% in 2005 and is expected to rise from roughly 12% in 1992 to 41% by 2022 within Saudi males, as well as women from 21% to 78% [7].
According to the WHO, obesity is defined as body mass index (BMI) ≥30 kg/m2, whereas overweight is defined as BMI ≥25 and < 30 kg/m2 [8]. According to the Framingham study, obese patients have a doubled risk of developing HF as compared to those with normal BMI. It has been found that for every 1 kg/m2 increase in BMI, the risk of HF increases by 7% in women and 5% in men [9].
The global burden of disease (GBD) recent estimations of obesity were found to be increasing in most countries; higher BMI accounted for approximately 4 million deaths in 2015; more than two-thirds of deaths were linked to cardiovascular diseases (CVD) [10, 11].
A recently published observational study from England and Scotland found that adiposity (overweight or obesity) attribution to all-cause deaths became ahead of smoking [12]. Furthermore, several metabolic abnormalities have been shown to be associated with obesity and overweight, which could ultimately give rise to serious diseases, such as CVD, diabetes mellitus (DM), hypertension (HTN), dyslipidemia, etc. [13].
Our article will provide a comprehensive overview of obesity-related HF, analyze existing obesity-related indices and parameters in relation to their influence on HF outcomes and prognoses, and identify key obesity-related pathogenesis and mechanisms influencing HF development. It will also highlight trends and gaps in this area, potential solutions, and recommendations for future investigation. Ultimately, our goal is to contribute to the advancement of knowledge in this area and provide valuable insights for clinical practice.
High waist circumference (WC) strongly predicts CVD risk [14, 15]. Other anthropometric indices of obesity, such as waist-to-hip ratio (WHR) and waist-to-height ratio, have been independently associated with HF incidents; however, indices such as WC and WHR have not been shown to perform better than BMI as predictors of HF [16].
BMI classification is a way of categorizing an individual’s weight status based on their BMI. The following are the standard BMI classifications, as defined by the WHO [17]:
- BMI < 18.5: Underweight
- BMI 18.5–24.9: Normal weight
- BMI 25–29.9: Overweight
- BMI 30–34.9: Obesity class I
- BMI 35–39.9: Obesity class II
- BMI ≥40: Obesity class III or morbid obesity
BMI cut points for Asians are slightly different from those for other populations. A BMI of 25 or higher is considered overweight for all populations by the WHO. However, for Asians, a BMI of 23 or higher is considered overweight, and a BMI of 25 or higher is considered obese [18]. Additionally, the International Diabetes Federation (IDF) recommends that Asians have a lower BMI cutoff point, with a BMI of 23 or higher indicating an increased risk for metabolic complications [19]. These lower cut points are based on evidence that Asians tend to have a higher percentage of body fat and a higher risk for obesity-related health issues at lower BMI levels than other populations.
WC is a better index of abdominal obesity than WHR and relates better with BMI than WHR [20]. A WC greater than 102 cm in men and greater than 88 cm in women indicates central obesity and increased cardiovascular (CV) risk [21]. At the same time, a WHR greater than 0.9 in men and greater than 0.85 in women indicates central obesity [22].
Although BMI is a common measure of body weight and obesity, it has some limitations. One limitation is that BMI does not distinguish between lean mass and fat mass, which can lead to misclassification of individuals with high muscle mass as overweight or obese. It may also not accurately reflect body fat distribution, an important predictor of health outcomes. For example, people with high levels of abdominal fat, even those with a normal BMI, may be at an increased risk of metabolic disorders such as DM and CVD. Further, BMI cut points may not reflect the health risks of certain ethnic groups, such as Asians, accurately as described above [23].
Due to its simplicity, low cost, and ease of use in large population studies, BMI remains a widely used measure of body weight and obesity. It remains a useful tool for identifying people at high risk for obesity-related health problems and for monitoring body weight changes over time. In order to provide a more comprehensive assessment of an individual’s health status, BMI must be considered along with other measures of body composition and health risk factors [18].
When it comes to measuring body fat, obesity and adiposity are two distinct concepts. Typically, obesity is defined as having a BMI of 30 or more, indicating excessive amounts of body weight relative to height. Adiposity, on the other hand, refers to the amount of body fat regardless of weight or BMI. It is well known that obesity is a risk factor for various health problems, including DM, CVD, and certain types of cancer, but adiposity does not necessarily indicate poor health outcomes [24]. In fact, some studies have suggested that having a higher proportion of body fat in certain areas, such as the hips and thighs, may be protective against certain chronic diseases [25]. Therefore, assessing an individual’s health status requires considering other measures of body composition, including adiposity.
The existence of cardiometabolic ailments and CVD in individuals with normal-weight obesity contributes to CVD risk misperception and misdiagnosis in clinical practice, particularly among patients with excess fat but not obesity as defined by BMI [26–28].
Individuals with normal-weight obesity have a normal body weight or BMI, but a high level of body fat. This condition is also known as “skinny fat” or “thin on the outside, fat on the inside” [27]. Normal-weight obesity is associated with metabolic disorders such as insulin resistance, dyslipidemia, HTN, and DM, as well as CVD [29]. Studies have shown that individuals with normal-weight obesity have a higher mortality rate than those with similar BMI but less body fat percentage [30].
Because new data supports visceral obesity as an indication of CV pathogenesis [31], experts propose assessing the WC in conjunction with BMI in clinical settings [26–28].
Body imaging using various imaging modalities has emerged, allowing for the quantification of the volume or area of different adipose tissue and ectopic fat (liver, pancreas, heart, and skeletal muscle) referred to as intra-abdominal or visceral adipose tissue (VAT) [32].
Individuals with low levels of VAT within the overweight and obese categories have a more favorable CVD risk profile, which is frequently referred to as metabolically healthy obesity [33, 34].
Epicardial adipose tissue (EAT) is a type of visceral fat that surrounds the heart and is associated with the development of CVD. BMI and EAT have been found to correlate positively, with individuals with higher BMI having higher EAT [35, 36]. This relationship is thought to be due to the fact that excess adiposity leads to an increase in proinflammatory cytokines released by adipose tissue, which can lead to EAT accumulation and CVD development [37]. In addition, EAT has been shown to be a better predictor of CV risk than BMI alone, suggesting that measuring EAT may be helpful in assessing CV risk in obese individuals [38].
According to research, EAT thickness is highly connected to WC, blood pressure, insulin resistance indicators, and dyslipidemia [39, 35]. Furthermore, epicardial fat thickness has been linked to sleep apnea severity in women regardless of BMI [40], and sleep apnea is related to an increased risk of CVD [41]. Exercise has been shown to lower VAT even in the absence of weight loss; a meta-analysis found that exercise resulted in a 6.1% reduction in VAT in the absence of weight loss [42].
EAT can be measured using imaging techniques such as echocardiography, CT, and MRI. These techniques provide a non-invasive and accurate assessment of EAT volume and thickness, which can be used to assess the risk of CVD for obese individuals [43].
Adiposity directly affects cardiac function through its effects on the myocardium and vasculature and indirectly through its impact on comorbid conditions (e.g., HTN, etc.) [44]. Adipose tissue accumulation leads to hemodynamic changes, such as higher blood volume, cardiac output (CO), and lower systemic vascular resistance (SVR) [45] (Figure 1). Increasing adiposity also leads to higher blood pressure because of neurohormonal activation and metabolic abnormalities, including renin-angiotensin-aldosterone system activation, sympathetic system activation, hyperleptinemia, and dysregulation of growth factors, such as insulin-like growth factor [46].
Enhancing the activity of the renin-angiotensin-aldosterone system leads to hypertrophy and apoptosis of cardiac myocytes and myocardial fibrosis [47].
With an increase in blood volume, venous backflow is enhanced, which causes an increase in ventricular preload, resulting in an increase in ventricular wall tension and, ultimately, a dilation of the ventricles.
Abdominal obesity is associated with subclinical left ventricular (LV) dysfunction [48]. Echocardiographic techniques such as 2-dimensional (2D) speckle tracking global longitudinal strain analysis permit early identification of LV systolic dysfunction despite preserved LV ejection fraction (EF) in asymptomatic individuals, particularly DM patients [49].
LV afterload increases with HTN, increasing the risk of heart structural and electrical remodeling. Ultimately, this process results in left ventricular hypertrophy (LVH) and diastolic and systolic dysfunction [50].
Inflammatory cytokines (TNF-α, IL-1, IL-6, IL-8, etc.) increase in obesity and play an essential role in the development of HF by causing myocardial fibrosis, which increases myocardial stiffness and may thereby lead to diastolic and later to systolic heart dysfunction [51–54].
As obesity advances, the hypertrophied adipocytes undergo apoptosis, cell necrosis, and fibrosis, which further induces a low-grade systemic proinflammatory state and adipose tissue dysfunction. Then, the inflammatory response is intensified by macrophage recruitment to adipose tissue, resulting in insulin resistance [55]. Insulin resistance reduces the contractility of the myocardium, increasing the chances of HF [56].
It is important to note that obesity also has a direct effect on the myocardium, causing myocardial fat accumulation and subsequent fibrosis, leading to the development of left ventricular diastolic dysfunction (LVDD) and heart failure with preserved ejection fraction (HFpEF) [57, 58].
Enhanced fibrosis and myocardial lipid accumulation can also contribute to developing cardiac arrhythmias, leading to HF [59, 60].
The alteration of lipid metabolism increases the risk of atherosclerosis and ischemic cardiomyopathy (CM). Therefore, obesity is an independent coronary artery disease (CAD) risk factor [45]. Atherosclerotic heart disease associated with obesity can cause heart failure with reduced ejection fraction (HFrEF). In addition, obesity-related comorbidities such as DM, sleep apnea, and hypoventilation syndrome can increase the risk of pulmonary hypertension (PHTN) and right ventricular (RV), and LV failure [44].
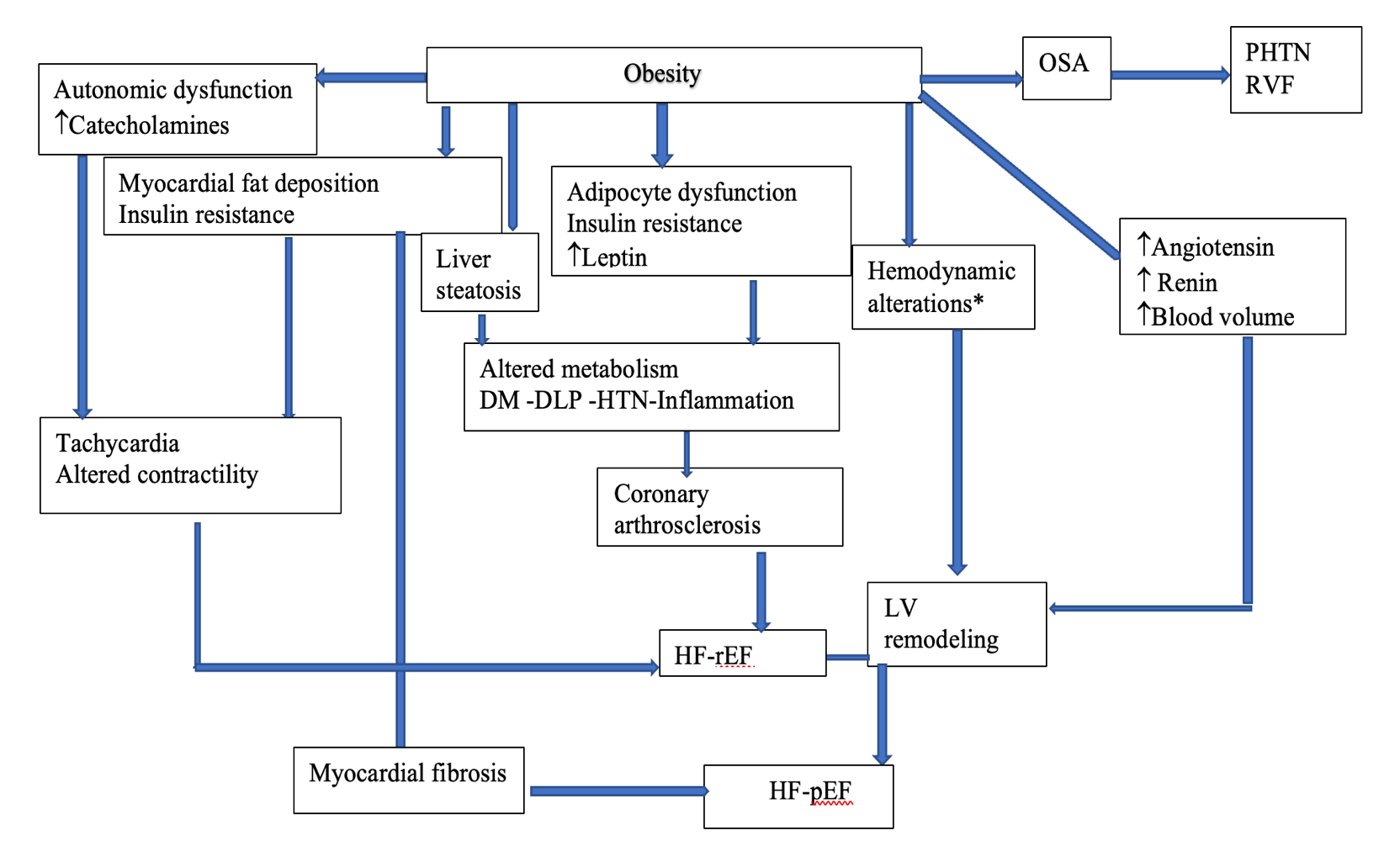 Figure 1: The various mechanisms in obese individuals that may lead to the development of heart failure. *Hemodynamic alteration: ↓SVR (systemic vascular resistance), ↑CO (cardiac output). OSA: obstructive sleep apnea; PHTN: pulmonary hypertension; RVF: right ventricular failure; DM: diabetes mellitus; DLP: dyslipidemia; HTN: hypertension; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; LV: left ventricular.
Figure 1: The various mechanisms in obese individuals that may lead to the development of heart failure. *Hemodynamic alteration: ↓SVR (systemic vascular resistance), ↑CO (cardiac output). OSA: obstructive sleep apnea; PHTN: pulmonary hypertension; RVF: right ventricular failure; DM: diabetes mellitus; DLP: dyslipidemia; HTN: hypertension; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; LV: left ventricular.
The frequency of HF is increasing; it is one of the significant causes of death globally, with a prevalence of approximately 3% in developed countries [61]. There have been numerous studies showing obesity to be a significant health concern causing HTN, CVD, and LVH, which all are vital risk factors for the development of HF [62, 63]. Morbid obesity correlates closely with HF development: after 20 years of obesity, the prevalence of HF increases by 70%, and after 30 years, it increases by 90% [64].
Obesity and overweight adversely affect cardiac structure and function (both systolic and diastolic function), increasing the prevalence of HF. Increasing preload causes a sustained rise in CO, causing an initial dilation of the LV, followed by hypertrophic compensation [65]. While obesity significantly increases the risk of HF, a recent study suggests the risk of HFpEF is higher with a higher BMI than HFrEF [66]. As obesity severity increases, LV mass grows concentric, often resulting in diastolic dysfunction [65]. The severity of obesity as measured by BMI was found to correlate with the risk of HF as class I obesity was reported to have a 12% prevalence of diastolic dysfunction, class II obesity 35%, and class III obesity 45% [67]. In our recently published observational study, we found a significant correlation between obesity and abnormal LV geometry accordingly concentric remodeling (CR) was the commonest among class I obesity (48.8%), followed by eccentric LVH (eLVH) (46.2%), and concentric LVH (cLVH) (31.7%), respectively and only (39.8%) have a normal geometry [68]. Pandey et al. [69] demonstrated that a higher BMI is associated with a higher risk of HFpEF, with overweight and class 1 obesity having an average of 38% and 56% higher chances, regardless of other CV risk factors.
In patients with obesity and HFpEF, cLVH, RV dilatation, and RV dysfunction were more pronounced. Furthermore, obese individuals with HFpEF had greater interventricular interdependence and pericardial restraint due to greater epicardial fat thickness and volume [57, 58]. As a result of the structural and functional changes observed in obesity, the heart’s function deteriorates, which is termed obesity cardiomyopathy [70]. These changes can be reversed with purposeful weight loss [71].
Despite the adverse effects of obesity on LV structure and function, several studies have demonstrated a robust relationship between obesity and prognosis, with overweight and obese class I and II HF patients having a better prognosis than their leaner counterparts [72, 73]. In obese patients, SVR is reduced, resulting in increased CO, which is a potential protective mechanism in patients with HF [65]. According to Oreopoulos et al. [74], overweight and obese patients with HF had a reduction in CVD mortality (19% and 40%, respectively) and all-cause mortality (16% and 33%, respectively) compared to HF patients without elevated BMI.
In HF, there is evidence of the obesity paradox: overweight or obese patients have better clinical outcomes than normal-weight patients with the same degree of HF, which is more noticeable in HFrEF than in HFpEF [62, 63, 75]. The protective effects of obesity on CV outcomes have now been noted for HFrEF, HFpEF, and acute decompensated HF [62, 63, 75, 76]. It has also been noted that obesity paradoxes exist for BMI, WC, and percent body fat [63, 75–78]. HF patients have lower EAT compared with the general population, and a recent study found that this is associated with higher HF mortality, another aspect of the obesity paradox [76, 79, 80]. The brain natriuretic peptide (BNP) level in obese patients, including those with HF, is lower than in patients with normal weight [62, 63, 76]. Weight loss after bariatric surgery leads to increased N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels with improved LVDD in patients with severe obesity [81]. Furthermore, extra adipose tissue and higher lean muscle mass may protect against cardiac cachexia and sarcopenia in advanced HF, which are associated with poor cardiac outcomes [82–84] (Figure 2).
In a meta-analysis study, the low (normal or underweight) BMI group was found to have the highest adverse events, mortality, and re-hospitalizations compared with the overweight group [85]. The studies show that higher BMI and WC are associated with better event-free survival in HF, with the best survival for those with increased BMI and high WC [77, 78]. Although obesity is associated with severe HF exacerbations, it is associated with lower in-hospital mortality rates [86]. A chronic catabolic state in HF results in fat mass and lean mass loss (i.e., cachexia), which will have devastating outcomes [73]. A significant association between advanced HF and cachexia may explain why heavier advanced HF patients demonstrate an obesity paradox, likely due to an increased metabolic reserve [65].
Furthermore, obese individuals have higher levels of circulating lipoproteins that may bind and detoxify lipopolysaccharides involved in releasing inflammatory cytokines [71]. Finally, obese patients may show improved cardiorespiratory fitness due to increased lean mass, which is associated with improved outcomes [87, 88].
On the contrary, a recently published study explodes the obesity-survival paradox theory, indicating measuring WHR rather than looking at BMI mitigates the survival advantage for people with a BMI of 25 kg/m2 or more. Therefore, dismissing weight-related indices and focusing on greater adiposity by WHR might better predict a higher risk of HF hospitalization. A venture which requires further substantiation [89].
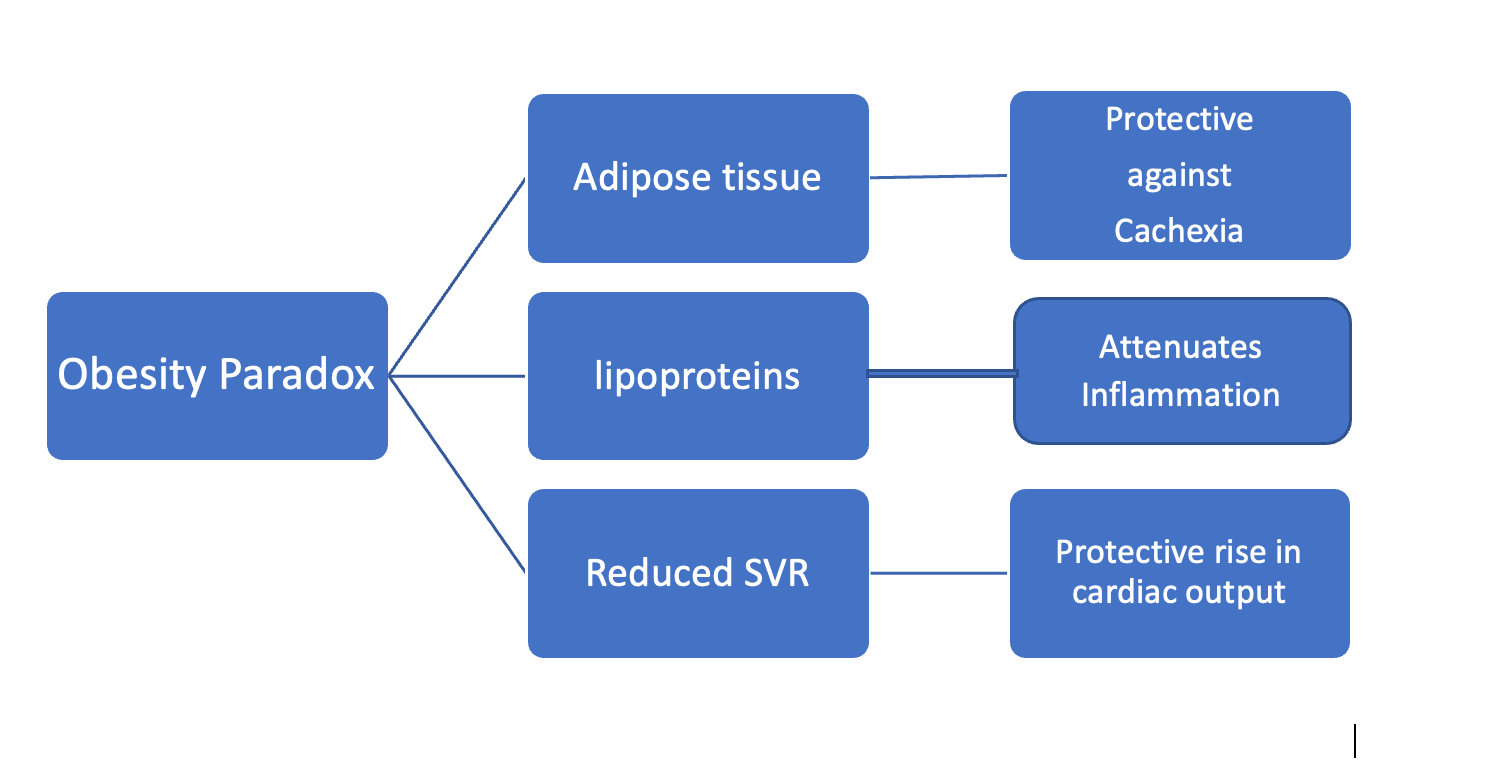 Figure 2: Postulated mechanism of heart failure paradox. SVR: systemic venous return.
Figure 2: Postulated mechanism of heart failure paradox. SVR: systemic venous return.
Losing weight intentionally may be one of the most effective long-term treatments for improving hemodynamics, cardiac structure, and functional abnormalities associated with obesity, most of which harm HF [72, 90]. While there is little evidence that weight loss in HF leads to better paramount clinical survival, weight loss may reduce symptoms and improve quality of life and other medical conditions such as sleep apnea or DM [76]. The major HF societies during the past decade have vastly different recommendations regarding intentional weight loss strategies, none of which recommended weight loss for HF patients. However, the agreed concept was the exitance of inferior prognosis in class III obesity [65].
The latest 2022 review of HF guidelines by the American College of Cardiology Foundation/American Heart Association (ACC/AHA) and the 2021 HF review by the European Society of Cardiology (ESC) recommended weight loss to prevent HF. Still, they did not recommend weight loss in obese patients with established HF due to the lack of large-scale studies examining the safety or efficacy of weight loss with diet, exercise, or bariatric surgery [91, 92]; in a study by Mahajan et al. [93], the obesity paradox was observed for all-cause mortality in obese patients regardless of improved cardiac structure and function following intentional weight loss.
Although current ACC/AHA and ESC guidelines do not recommend weight loss in established HF, they recommend exercise training, in addition to lifestyle modifications (Table 1) and regular physical activity (PA), as safe and effective ways to improve functional status in patients with HF [91, 92]. This is based on a meta-analyses study which indicates that cardiac rehabilitation can reduce mortality, improve functional capacity, exercise duration, and quality of life and reduce hospitalizations [94].
High fitness and PA strongly influence the prognosis of patients with established HF [66, 69, 95]. It has been shown in several studies that patients with HF with preserved fitness levels have a very good prognosis, regardless of their BMI [62, 63, 76, 96–98]. In the Physicians Health Study, in addition to increasing BMI, a lower level of PA was also associated with an increased risk of HF, with the highest relative risk of HF seen in obese men who were also physically inactive compared to men who were lean and physically active [99]. Therefore, for people with obesity and HF, PA and exercise training are highly recommended, especially for fitness improvement.
| Lifestyle modifications | AHA/ACC | ESC |
| Salt restriction | Avoid in stage C HF | Avoid > 5 gm/day |
| Specific diet | Mediterranean-DASH | NON |
| Fluid restriction | + | + |
| Weight loss | NON | Pre-HF transplant BMI < 35 kg/m2 |
| Exercise | Cardiac rehabilitation | Regular exercise |
Table 1: ACC/AHA and ESC recommendations for lifestyle modification. AHA: American Heart Association; ACC: American College of Cardiology; ESC: European Society of Cardiology; HF: heart failure; BMI: body mass index.
Several medications are currently available for weight loss, among them orlistat, a lipase inhibitor, which has limited efficacy and safety for treating obesity with HF [100, 101]. Some new classes of drugs that were first created for people with type 2 DM are promising in treating obesity and HF. The efficacy of a glucagon-like peptide agonist (liraglutide) has been demonstrated in HF [102–104]. However, its efficacy as a weight loss agent among patients with HFrEF and a recent acute HF hospitalization remains unknown. Another agent is sodium-glucose co-transporter 2 (SGLT2) inhibitors for weight loss and reducing hospitalization for HF and CV death [105].
Weight loss from SGLT2 inhibitor medication is hypothesized to be one of the processes implicated in reducing mortality because of the increased glucagon:insulin ratio, which causes significant lipid mobilization [106, 107]. Weight reduction of up to 2.7 kg was found in type 2 DM patients treated with SGLT2 inhibitor medication, while other studies suggest weight loss in pre-diabetic patients [108, 109]. There is currently no evidence that it may result in weight loss in individuals with HF who lack diabetes, which would argue against weight loss being the critical mechanism of benefit with SGLT2 inhibitor medication.
Furthermore, despite the high prevalence of obesity in HF, there is little conclusive information on the effect of weight loss on cardiac function, quality of life, and exercise tolerance in HF patients [110]. As a result, weight loss alone cannot explain the benefits of SGLT2 inhibition in HF. Several clinical trials are underway to investigate the causes of CV advantages associated with SGLT2 inhibition, including the impact on cardiac remodeling, lipolysis and change of epicardial fat thickness, and endogenous ketone generation. These researches will shed light on the critical mechanisms involved in the cardioprotective benefits of SGLT2 inhibitor medication.
![]() *1,2, Husain MA
*1,2, Husain MA![]() 2, AlQahtani A
2, AlQahtani A![]() 1 and Al Qarni A
1 and Al Qarni A![]() 1,3,4,5
1,3,4,5

