Abstract
Background: All over the world, hundreds of millions of population suffer from common bacterial infections of the urinary tract every year. An increased risk of death, morbidity, and increased healthcare expenses in the critical care unit is linked to catheter-associated urinary tract infections (CAUTIs), which occur when bacteria enter the urinary tract through a urinary catheter. Since urinary catheterization is linked to urinary tract infections (UTIs), which are among the most common infections in healthcare settings and account for almost 30% of intensive care unit (ICU) reports, there is a significant opportunity for prevention.
Methods: An institutional-based cross-sectional study design is applied to determine the prevalence and associated factors of healthcare-associated urinary tract infections (HAUTIs) among selected adult patients accounting for 391 that were admitted to ICU in the two years at Addis Ababa Public Governmental Hospital, Addis Ababa, Ethiopia, from June–December 2020. Data was manually checked and entered into EpiData Manager version 4.6.1, and statistical analyses were performed using the SPSS version 23 software program. The strength of the association between dependent and independent variables is assessed using crude odds ratio (COR) and adjusted odds ratio (AOR) with a confidence interval (CI) of 95%. Variables with a value of P < 0.25 on bivariate analysis were directly forwarded to be analyzed by multivariable analysis. The goodness of the FIT test was also computed for logistic regression using the Hosmer-Lemeshow test, resulting in (sig = 0.073); finally, having P-values < 0.05 is considered statistically significant.
Results: The study finds that the prevalence of HAUTI among ICU admitted patients was 91 (23.3%) 95% CI; (19.2-27.4), while the length of stay, having tracheostomy, patients on mechanical ventilation, and taking proton pump inhibitor (PPI) drugs were associated with HAUTI in the study area.
Conclusion: HAUTI is a highly emerging clinical condition among ICU-admitted patients in the study areas.
Keywords
healthcare-associated urinary tract infections, intensive care unit, Addis Ababa, Ethiopia
Abbreviations
CAUTIs: catheter-associated urinary tract infections, UTIs: urinary tract infections, ICUs: intensive care units, HAUTIs: healthcare-associated urinary tract infections, HAIs: healthcare-associated infections, COR: crude odds ratio, AOR: adjusted odds ratio, CI: confidence interval, ECDC: European Centre for Disease Prevention and Control, SPHMMC: St. Paul’s Hospital Millennium Medical College, AABET: Addis Ababa Burn, Emergency and Trauma, GCS: Glasgow Coma Scale, CHF: congestive heart failure, PPIs: proton pump inhibitors
Introduction
A catheter-associated urinary tract infection (CAUTI) arises when the infectious agent enters the urinary tract via a urinary catheter, leading to infection, and has been associated with increased mortality, morbidity, and healthcare costs in intensive care units (ICUs) [1]. It affects any anatomic part of the urinary system, including the kidney, ureter, bladder, and urethra [2].
Although healthcare-associated infections (HAIs) constitute a major endangerment in all healthcare settings, their incidence is higher in patients in critical care units than in any other wards. In developed countries, 5–15% of hospitalized patients and more than 50% of patients in ICUs develop HAIs. In resource-limited countries, the magnitude of HCAIs is underrated or unknown due to the nonexistence of a well-established infection surveillance system [3]. Patients admitted to the ICU are vulnerable to HAIs owing to poor body defense, seriousness of underlying disease, deprived nutritional status, frequent exposures to invasive devices, and exposure to an extensive range spectrum of drugs [4].
Internationally, over 150 million people are affected by common bacterial infections called urinary tract infections (UTIs). UTIs are caused by wide-ranging pathogens, comprising gram-negative and gram-positive bacteria as well as fungi. The most common causal agent for uncomplicated and complicated UTIs is uropathogenic Escherichia coli [5].
UTI is one of the highest prevalent healthcare-related infections, accounting for nearly 30% of ICU reports of all HAIs because of its association with urinary catheterization, but has great preventive potential. Nosocomial UTIs have been associated with a threefold increased risk for death in hospital-based studies, with estimates of more than 50,000 excess deaths occurring per year in the USA as a result of these infections [6].
During hospitalization, indwelling urethral catheters account for about 80% of UTIs. Urinary catheters may aid colonization of the urinary bladder due to wrong catheter placement, extended stay of catheterization, applied in septic technique, poor hand hygiene, and septic way of the urethral orifice opening [7]. Indeed, as reported by European Centre for Disease Prevention and Control (ECDC), the urinary catheter utilization rate was 78 per 100 patient days in ICUs, and nearly 98% of UTIs were associated with the presence of a urinary catheter [5]. Prolonged catheterization, lengthened ICU stay, being female gender, older age, catheter insertion outside operating theatre, patients diagnosed with sepsis, diabetic patient, poor immunity or immune-compromised patients, urology service of the hospital, presence of other active sites of infection, poor nutrition, raised creatinine, application of ureteric stents, rough monitoring of urine output, improper positioning of drainage tube, and improper antimicrobial drug therapy [2, 6, 8].
Serious sequelaes such as recurrences, gram-negative bacteremia, pyelonephritis, renal damage, preterm birth endocarditis, vertebral osteomyelitis, septic arthritis, colitis cystitis, and complications caused by repeated antimicrobial use like antibiotic resistance, Clostridium species, meningitis [9]. These all lead to discomfort for the patient, with an extension of the mortality rate of 23 deaths per 1000 inpatients and excess costs of $1000/case [10].
The US Centers for Disease Control and Prevention (CDC) strongly recommends using soapy water and distilled water or Povidone-iodine for sterilizing the periurethral area before catheterization is performed. However, some studies have reported that this approach was not effective in decreasing the UTI rate [11]. Limiting the duration of catheterization, avoiding frequent and unnecessary catheterization, following standard infection control protocols, implementing sterile precautions during catheter insertion, and finally, preservation of indwelling urinary catheters can reduce catheter-related complications.
Materials and Methods
2.1 Study design, objective, population, area and period
An institutional-based retrospective cross-sectional study was conducted to determine the prevalence and associated factors of healthcare-associated urinary tract infection (HAUTI) among adult patients admitted from 2017 to 2019 to the ICU of Addis Ababa Governmental Hospitals from June–December 2020.
2.2 Inclusion and exclusion criteria
Adult patients admitted to Addis Ababa Governmental Hospital ICUs from 2017 to 2019 who stayed above 48 hours of ICU admission were included, while incomplete or ambiguous charts and having prior diagnosed infection were excluded from the study.
2.3 Sample size determination
To calculate the sample size to determine the prevalence and associated factors of HAUTI in the two hospitals was done by a single proportion formula:
n = z2 p (1-p) / d2
Where n = sample size; d = maximum allowable error (margin of error) = 0.05; z = value of the standard normal distribution (z-statistic) at 95% confidence level (z = 1.96); p = expected prevalence (proportion) since, in our country, there is no specific ICU study regarding the prevalence and associated factors of HAUTIs, a p-value of 0.5 is considered.
n = (1.96)2 × 0.5 (1-0.5) / (0.05)2 = 384, then 5% of the sample size is considered for incomplete charts and unavailable (contingency), 384 (0.05) results 19.2.
The total sample size will be 384 + 19.2 = 404.
2.4 Sampling techniques
St. Paul’s Hospital Millennium Medical College (SPHMMC) was selected by lottery method among all federal hospitals found in Addis Ababa and its affiliated Addis Ababa Burn, Emergency and Trauma (AABET) Hospital was included and studied. After the proportional allocation of 187 and 217 individual charts to SPHMMC and its affiliated AABET Hospital, a systematic random sampling technique was employed to select ICU cases in the two years, which means after arranging patients charts sequentially, the first patient chart taken as case one by lottery method from the first three patient medical charts. Then, the other study subjects were selected and included in every three client chart intervals.
2.5 Operational and term definitions
Healthcare-associated urinary tract infection (HAUTI)- is defined as either a microbiologically confirmed symptomatic UTI or a non-microbiologically confirmed symptomatic UTI developed after 48 hours of ICU admission.
Catheter-associated urinary tract infection (CAUTI)- is a HAI developed after the insertion of a urinary catheter, central line catheters, and endotracheal or tracheostomy tube to the body either in intermittent or continuous ways within the 48-hour period before the onset of infection.
Multidrug resistance (MDR)- an identified bacteria that is not susceptible to at least one antimicrobial agent in three or more antimicrobial groups.
2.6 Data collection technique
Data was collected from April 01 to April 22, 2020, by two trained health professionals from patients’ medical records using a slightly modified checklist adopted from previous studies. Concurrently, the principal investigator observer clarifies and evaluates the whole data collection process and examines proper ethical procedures. HAUTI case definitions were adapted from the ECDC reporting format, and both clinical signs and symptoms of patients and laboratory (culture) results were used.
2.7 Data quality management
Data collectors were trained, and the principal investigator continuously observed, made clarifications, and supervised the whole activities of the study. Then, the collected data was checked for completeness and consistency and loaded into statistical software in the safest way for both data cleaning and analysis.
2.8 Data processing, analysis, presentation and interpretation
After proper data collection, the data was checked for completeness, then coded and entered into EpiData Manager version 4.6.1 by the principal investigator. Then, it is exported to SPSS Statistics version 23 for mainly analysis and cleaning.
Descriptive statistics of the result were expressed by frequency, percentages (cross-tabulation), and median along the interquartile range. Normality test has been done using Kolmogorov-Smirnov and Shapiro-Wilk test. A multicollinearity test (variable inflation factor = 1.00) was also done to examine similarities between the independent variables.
The goodness of the FIT test also worked out for logistic regression using the Hosmer-Lemeshow test, resulting in (sig = 0.073). The risk factors were determined using bivariate and multivariate binary logistic regressions. Variables with a value of P < 0.25 on bivariate analysis were directly forward to be analyzed by multivariable analysis; having P-values < 0.05 is considered statistically significant. The strength of association was assessed using crude odds ratio (COR) and adjusted odds ratio (AOR) with a confidence interval (CI) of 95%. Tables, charts, and texts were preferred for data presentation.
2.9 Ethical consideration
Ethical clearance was obtained from SPHMMC after being informed by a written letter from the ECCN department; then, permission was also taken from both (SPHMMC and AABET) hospital administrations to conduct the study after submitting an ethical approval letter. Then, data was collected from patient charts in a way that made it impossible to identify using newly assigned code numbers, and questionnaires were kept in a safe place.
Findings of the Study
3.1 Socio-demographic status of the participants in Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia, 2020
From the total sample size, 391 (97%) patients were included in the study, and the rest of the participants’ charts were unavailable during the collection. The age of the patients ranged between 18 and 94 years, and merely half (50.1%) of the participant’s age were grouped between 18–34 years. 34 years was the median age of the participants, with an interquartile range of plus or minus 30 years.
Nearly half of the research participants, 209 (53.5%), were male, with male to female ratio of 1: 0.87. Nine days was the median ICU length of stay with an interquartile range of 15 days. Patients had been admitted to the ICU between 3 and 104 days. Emergency outpatient was the most referred hospital department 199 (50.9%), followed by medical ward 70 (17.9%). Only 84 (21.5%) participants had previous admission history (Table 1).
| Variable | Category | HAUTI | Total (%) |
| | | No (76.7%) n = 300 | Yes (23.3%) n = 91 | n = 391 |
Sex | Male | 157 (52.3) | 52 (57.1) | 209 (53.5) |
| Female | 143 (47.6) | 39 (42.8) | 182 (46.5) |
| Age group of participants | 18–34 | 147 (49) | 49 (53.8) | 196 (50.1) |
| 35–55 | 83 (27.6) | 18 (19.8) | 101 (25.9) |
| ≥56 | 70 (23.3) | 24 (26.4) | 94 (24) |
Length of stay in ICU | 3–9 days | 167 (55.7) | 17 (18.7) | 184 (47.1) |
| ≥9 days | 133 (44.3) | 74 (81.3) | 207 (53) |
| Department before to ICU | Emergency OPD | 155 (51.7) | 44 (48.2) | 199 (50.8) |
| Medical ward | 53 (17.7) | 17 (18.6) | 70 (17.9) |
| Surgical ward | 29 (0.1) | 16 (17.0) | 45 (11.5) |
| Obstetrics and gynecology | 41 (13.5) | 6 (0.1) | 47 (12) |
| Others sites | 22 (0.1) | 8 (0.1) | 30 (76.8) |
Previous admission history | Yes | 68 (22.7) | 16 (17.6) | 84 (21.5) |
| No | 232 (77.2) | 75 (82.3) | 307 (78.5) |
Table 1: Socio-demographic status of the participants in the two-year period, Addis Ababa, Ethiopia.
3.2 Clinical characteristics of the study participants at Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia
Respiratory failure 171 (43.7%), traumatic brain injury 86 (22%), and acute kidney injury 86 (22%) were the most common underlying disease conditions in the study areas.
About 236 of the participant’s baseline Glasgow Coma Scale (GCS) was above 8. About 77.7% of participants had received prophylaxis antibiotics, 279 (71.4%) study population were intubated, and only 36 (9.2%) had chest tubes. Merely 12 (1.3%) participants had central vascular catheters. More than two-thirds of 327 (83.6%) of admitted patients had indwelling urinary catheters in situ (Figure 1 and Table 2).
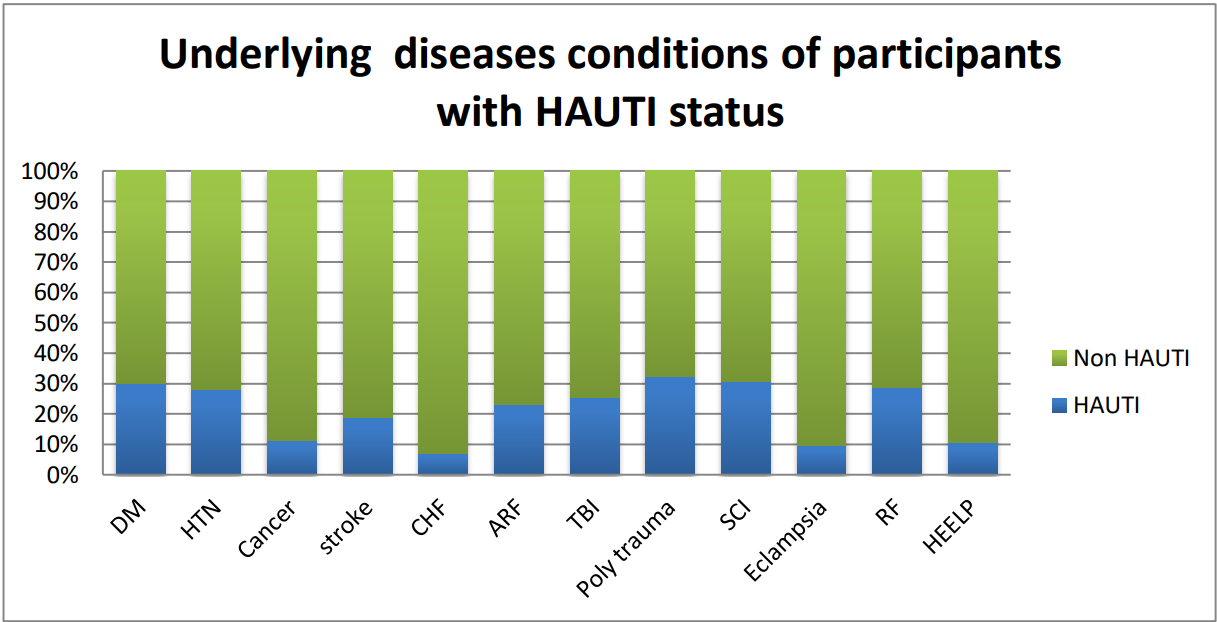
Figure 1: Common underlying diseases with HAUTI status among adult ICU participants in Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia, 2020. DM: diabetes mellitus; HTN: hypertension; CHF: congestive heart failure; ARF: acute renal failure; TBI: traumatic brain injury; SCI: spinal cord injury; RF: respiratory failure; HELLP: hemolysis elevated liver enzyme low platelets.
| Variable | Category of variable | HAUTI | Total (391) |
| | Yes (91) n (%) | No (300) n (%) | N (%) |
| GCS | 3–8 | 44 (48.4) | 111 (37) | 155 (39.7) |
| 9–15 | 47 (51.6) | 189 (63) | 236 (60.3) |
| Central vascular catheter | Yes | 21 (23.0) | 51 (17) | 72 (18.4) |
| No | 70 (77) | 249 (83) | 319 (81.6) |
| Endotracheal tube | Yes | 85 (93.4) | 194 (64.6) | 279 (71.3) |
| No | 6 (6.6) | 106 (35.4) | 112 (28.6) |
| Urinary catheter | Yes | 87 (95.6) | 240 (8) | 327 (83.6) |
| No | 4 (4.4) | 60 (92) | 64 (16.4) |
| Mechanical ventilator | Yes | 85 (93.4) | 195 (65) | 280 (71.6) |
| No | 6 (6.6) | 105 (35) | 111 (28.4) |
| Tracheostomy | Yes | 38 (41.7) | 24 (8) | 62 (15.9) |
| No | 53 (58.3) | 276 (92) | 329 (84.1) |
| Nasogastric tube | Yes | 84 (92.3) | 208 (69.3) | 292 (74.6) |
| No | 7 (7.7) | 92 (30.7) | 99 (25.4) |
| Chest tube | Yes | 13 (14.2) | 23 (7.6) | 36 (9.2) |
| No | 78 (86.8) | 277 (92.4) | 355 (90.8) |
| Surgery within a month | Yes | 50 (54.9) | 135 (45) | 185 (47.3) |
| No | 41 (46.1) | 165 (55) | 206 (52.7) |
| Received prophylaxis antibiotics | Yes | 84 (92.3 | 220 (73.3) | 304 (77.8) |
| No | 7 (7.7) | 80 (27.7) | 87 (33.2) |
| Blood transfusion | Yes | 30 (32.9) | 118 (39.3) | 148 (37.9) |
| No | 61 (68.1) | 182 (60.7) | 243 (62.1) |
| Immune-suppressant | Yes | 43 (47.2) | 117 (39) | 160 (41) |
| No | 48 (52.8) | 183 (61) | 231 (59) |
| Sedative drugs | Yes | 57 (62.6) | 117 (39) | 174 (44.5) |
| No | 34 (38.4) | 183 (61) | 217 (55.5) |
Proton pump inhibitor drugs | Yes | 52 (57.1) | 148 (49.3) | 200 (51.1) |
| No | 39 (42.9) | 152 (50.7) | 191 (48.1) |
Table 2: Clinical characteristics of the study participant at Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia, 2020.
3.3 Prevalence of healthcare-associated urinary tract infections with categories at Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia
A total of 91 (23.3%), 95% CI (19.2-27.4) study participants had been diagnosed with HAUTIs in the study areas. Approximately 87 (95.60%) of HAUTIs were associated with urinary catheters (CAUTI).
3.4 Identified pathogens of healthcare-associated urinary tract infection in the two-year period at Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia
Almost 14.8% of participants’ culture tests had been performed, and 50 bacteria (microorganisms) had been identified. Of recognized microbes, about 86% were gram-negative bacteria. Acinetobacter spp. (37%) were the highest number of identified microbes, followed by Klebsiella spp. (16%), E. coli (11%), Citrobacter and Proteus spp. (11%) were found to be highly prevalent causative agents of HAIs. About 72% of isolated bacteria were resistant to more than one antimicrobial (Figure 2).
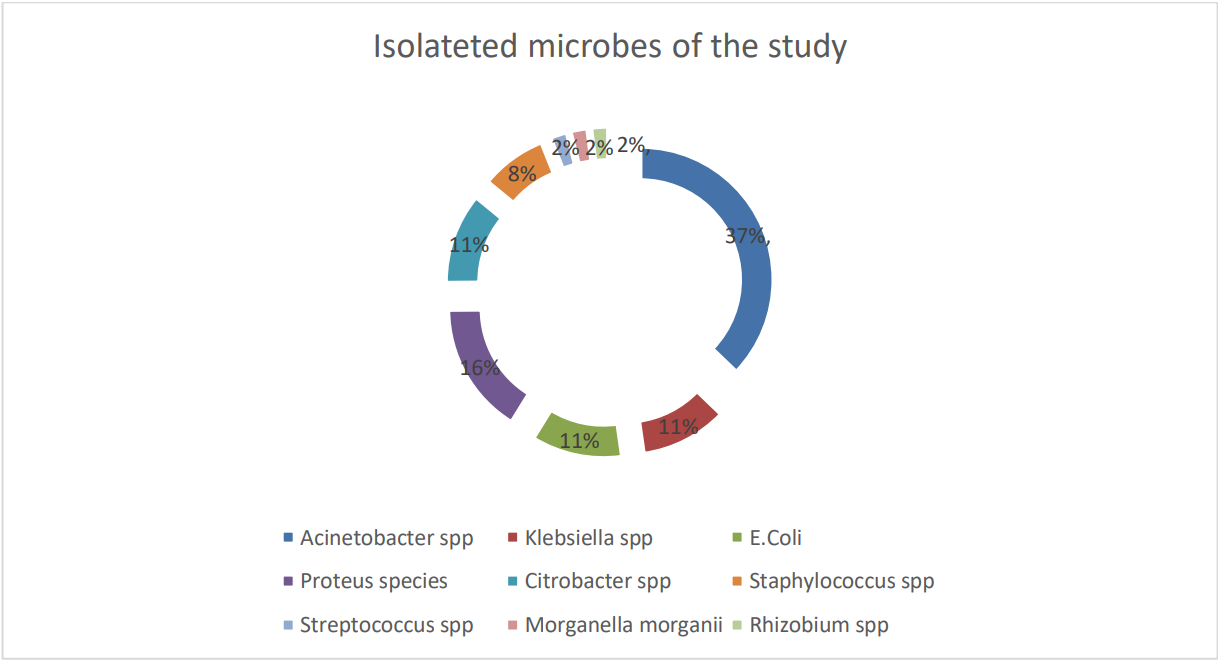
Figure 2: HAUTI distribution within isolated microorganisms from various specimens among adult ICU patients at Addis Ababa Public Government Hospital, Ethiopia, 2020 (n = 50).
3.5 Determinants of healthcare-associated urinary tract infections among adult ICU patients at Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia
On bivariate logistic regression analysis, the following variables were found to be statistically significant with HAUTIs. These variables were age, length of stay, prior ICU admission, cancer, congestive heart failure (CHF), polytrauma, eclampsia, respiratory failure, GCS, central vascular catheter, endotracheal intubation, indwelling urinary catheter, tracheostomy, chest tube, nasogastric tube, surgery within a month, received prophylaxis antibiotics, taking immunosuppressant drug, taking sedative agents, proton pump inhibitor (PPI), and blood transfusion.
However, in multivariate binary logistic regression, four variables were obtained to have a significant association with HAUTI. These were length of stay, patients with tracheostomy, patients who took PPIs, and patients who were on mechanical ventilation.
As the patient’s length of stay rises by a day, the possibility of acquiring infection was increased by 1.032 (AOR = 1.032, 95% CI (1.016, 1.049), P < 0.001). Participants with tracheostomy were five times at a higher risk for HAUTI (AOR = 5.33, 95% CI (2.29, 12.41) P < 0.001).
Patients who were on mechanical ventilation were six times more expected to acquire UTI (AOR = 5.61, 95% CI (1.06-29.65), P = 0.043), and taking PPI had two times higher possibility of getting HAUTIs (AOR = 2.04, 95% CI (1.03, 4.5), P = 0.041) (Table 3).
| Characteristics | HAUTI | COR (95% CI) | P-value | AOR (95% CI) | P-value |
| | Yes | No | | | | |
| Length of stay in ICU (days) | 91 | 300 | 1.05 (1.03-1.06) | 0.001 | 1.03 (1.02, 1.05) | 0.001 |
| Tracheostomy |
| Yes | 11 | 21 | 8.25 (4.57-14.86) | 0.001 | 5.33 (2.28, 12.41) | 0.001 |
| No | 188 | 171 | 1 | | 1 | |
| Mechanical ventilation |
| Yes | 85 | 195 | 7.63 (3.22-18.05) | 0.001 | 5.61 (1.06-29.65) | 0.043 |
| No | 6 | 105 | 1 | | 1 | |
| Proton pump inhibitors |
| Yes | 52 | 148 | 1.37 (0.85-2.19) | 0.193 | 2.04 (1.028-4.05) | 0.041 |
| No | 39 | 152 | | | | |
Table 3: Determinants of HAUTIs among patients admitted in Addis Ababa Public Governmental Hospitals, Addis Ababa, Ethiopia, 2020.
Discussion
The overall prevalence of hospital-acquired UTI in the two hospital ICUs was 91 (23.3%), 95% CI (19.4-27.6), and about 95.6% were associated with urinary catheterization. Under possibly assessed journals, the study finding is the first prevalence report in our country, which is particular to ICU. This prevalence of HAUTIs in the study area is much higher than the findings of 6.5% in a Canadian survey [6], Oumer et al. 16.8% [10], Saleem et al. 6.4% [12].
The higher prevalence result might be due to the consequence of inadequately trained health professionals and inadequate adherence to infection control measures [13]. Also, the nature of the study participants in the ICU may have reduced body defense, severe comorbid diseases, poor nutritional status, many times exposure to invasive devices, extensive range spectrum of drugs, and it is also stated that trauma patients tend to present with higher prevalence.
This could be due to ICU service and setting differences, dissimilar management and follow-up approaches, severity of comorbid illness, and participants’ diverse nature rate. Gram-negative bacteria (86%), specifically Acinetobacter spp. (37%), Klebsiella spp. (16%), E. coli (11%) and Proteus spp. (11%) were the main infectious agents in the study, which is in line with the South Ethiopian Study [10], Saudi Arabia [12], Ugandan [14], Alqarni [1], Fiji [15], and Romanian [16] ICU studies. The most dominant bacteria species in this study, Acinetobacter, commonly originated from water supplies of hospitals and contaminated materials [14].
From isolated microbe, the rate of multidrug-resistant bacteria (78%) was very high as compared to the Ugandan study (55.8%) and Nigerian study (57.1%) [14, 17], and this may be attributed to the very poor way of devise insertion and maintaining. It approaches the findings of the study done in Southern Ethiopia (88.1%), which may be due to the similarity found in both regions.
As the patient’s length of stay increases by a day, the likelihood of acquiring infection increases by 1.03 (AOR = 1.03, 95% CI (1.02, 1.05), P < 0.001). It is supported by Oumer et al. [10], the WHOs HAIs report [18], and Wang et al. [19]. The similarities as the length of stay increases, there would be an increment of exposure to the hospital environment, exposure to numerous invasive lines including urinary catheters, and worsening of comorbidities that increase the susceptibility of acquiring infection.
Patients with tracheostomy were at five times higher risk for developing HAUTIs (AOR = 5.33, 95% CI (2.28, 12.41), P < 0.001, which is in line with the study done in Thailand [20]. The finding may be explained as patients with tracheostomy tend to stay longer in the ICU, which may increase exposure to an infection. The tube may restrict their sensory and motor skills and lessen their range of motion as well as suppress their gag and cough reflex, which might disrupt them from safeguarding the airway, which may cause aspiration, and infection risk may increase from surgical procedures.
Participants with mechanical ventilation were 5.61 times at higher risk of acquiring HAIs (AOR = 5.61, 95% CI (1.06-29.65), P = .043). This is almost similar to the findings of Agaba et al. and Shao et al. [14, 21]. Mechanical ventilation, either through endotracheal or tracheostomy tube, increases the risk of infection from improper procedural application and predisposes the hematogenous spread of infection to different areas, including the urinary tract.
Patients who took PPIs were 3 times more likely to acquire infection in the hospitals (AOR = 2.04, 95% CI (1.028-4.05), P = 0.041). This may be PPI mechanistically, lessening of gastric acidity may lead to amplified gastric passage of pathogens or viable exogenous drug-resistant strains, delayed gastric emptying, increased bacterial translocation, and dysbiosis, resulting in intestinal colonization or infection [22]. This condition may increase the risk of infection, especially hospital-acquired pneumonia (HAP), which leads to UTI through hematogenous spread.
Conclusion
It is established that the prevalence of HAUTI is high in the study areas. Length of stay, having tracheostomy, mechanical ventilation use, and taking PPIs were determinants of HAUTIs in the study areas.
Recommendations
For the Federal Ministry of Ethiopia
HAI rate is one of the most vital indicators of quality of care. It is better to reinforce routine national surveillance of HAIs, especially HAUTIs, and further monitor implementation practices of alleviating HAUTIs, simultaneously encouraging emphasis on infection prevention and control practices.
For Addis Ababa Public Government Hospitals
It is suggested to evaluate evidence-based practices regarding mechanical ventilation, drug administration, and tracheostomy care. Furthermore, by facilitating investigation and management processes, it is better to shorten patient’s length of stay in hospitals, especially in ICUs.
Patients having tracheostomy and mechanical ventilation may require strict aseptic technique, evidence-based practice, and further daily monitoring afterward.
For health professionals
Since the rate of HAUTIs may show the quality of comprehensive care, it is necessary for intensive care nurses, physicians, and other professionals working on patient care to build up their role in infection prevention practices.
For researchers
It is crucial to conduct further studies to identify other key determinants of HAUTIs that might be better to conduct prospective studies. Also, it is recommended to observe the cause-effect relationship of some outlandish findings, like a higher likelihood of acquiring UTI among PPIs.
References
- Alqarni MS. Catheter-Associated urinary tract infection (CAUTI) in ICU patients. Middle East Journal of Nursing. 2021;15(1):25-33.
- Teshager T, Hussien H, Kefyalew M, et al. Knowledge, practice and associated factors of nurses towards prevention of catheter-associated urinary tract infection in intensive care unit of public hospitals administered by Federal Government in Addis Ababa, Ethiopia: a cross-sectional institutional-based study. BMC Nurs. 2022;21(1):186.
- Chernet AZ, Dasta K, Belachew F, et al. Burden of Healthcare-Associated Infections and Associated Risk Factors at Adama Hospital Medical College, Adama, Oromia, Ethiopia. Drug Healthc Patient Saf. 2020;12:177-85.
- Genaneh W, Sibhat M, Techane T, et al. Health care-associated infections and associated factors among adult patients admitted to intensive care units of selected public hospitals, Addis Ababa, Ethiopia. Int J Afr Nurs Sci. 2023;18:100570.
- Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-84.
- Laupland KB, Bagshaw SM, Gregson DB, et al. Intensive care unit-acquired urinary tract infections in a regional critical care system. Crit Care. 2005;9(2):R60-5.
- Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel). 2010;1(3):413-26.
- Parida S, Mishra SK. Urinary tract infections in the critical care unit: A brief review. Indian J Crit Care Med. 2013;17(6):370-74.
- CR, Dhakal B. Knowledge, Attitude and Practice on Prevention of Catheter-associated UTI among Nurses of a Tertiary Care Hospital. Journal of College of Medical Sciences-Nepal. 2021;17(1):61-8.
- Oumer Y, Regasa Dadi B, Seid M, et al. Catheter-Associated Urinary Tract Infection: Incidence, Associated Factors and Drug Resistance Patterns of Bacterial Isolates in Southern Ethiopia. Infect Drug Resist. 2021;14:2883-894.
- Liu Y, Xiao D, Shi XH. Urinary tract infection control in intensive care patients. Medicine (Baltimore). 2018;97(38):e12195.
- Saleem M, Syed Khaja AS, Hossain A, et al. Catheter-Associated Urinary Tract Infection in Intensive Care Unit Patients at a Tertiary Care Hospital, Hail, Kingdom of Saudi Arabia. Diagnostics (Basel). 2022 ;12(7):1695.
- Atici S, Soysal A, Kepenekli Kadayifci E, et al. Healthcare-associated infections in a newly opened pediatric intensive care unit in Turkey: Results of four-year surveillance. J Infect Dev Ctries. 2016;10(3):254-59.
- Agaba P, Tumukunde J, Tindimwebwa JVB, et al. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):349.
- Naidu K, Nabose I, Ram S, et al. A descriptive study of nosocomial infections in an adult intensive care unit in Fiji: 2011-12. J Trop Med. 2014;2014:545160.
- Mihaly V, Orsolya B, Monica O, et al. The Incidence and Risk Factors of Nosocomial Infections in ICU. Acta Marisiensis – Seria Medica. 2016;62(3):304-08.
- Iwuafor AA, Ogunsola FT, Oladele RO, et al. Incidence, Clinical Outcome and Risk Factors of Intensive Care Unit Infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. PLoS One. 2016;11(10):e0165242.
- Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organization. 2011.
- Wang L, Zhou KH, Chen W, et al. Epidemiology and risk factors for nosocomial infection in the respiratory intensive care unit of a teaching hospital in China: A prospective surveillance during 2013 and 2015. BMC Infect Dis. 2019;19(1):145.
- Manosuthi W, Thientong V, Moolasart V, et al. Healthcare-Associated infections at selected hospitals in thailand. Southeast Asian J Trop Med Public Health. 2017;48(1):204-12.
- Shao LW, Ni LM, Gao CH, et al. The incidence and risk factors of nosocomial infections in intensive care unit in China: an epidemiological study of 1718 patients. Int J Clin Exp Med. 2016;9(12):23642-3649.
- Willems RPJ, Schut MC, Kaiser AM, et al. Association of Proton Pump Inhibitor Use With Risk of Acquiring Drug-Resistant Enterobacterales. JAMA Netw Open. 2023;6(2):e230470.
![]() *1, Nega T2, Argeta H1, Gashaw S3, Shimeles E4, Sibhat M
*1, Nega T2, Argeta H1, Gashaw S3, Shimeles E4, Sibhat M![]() 5, Girma T
5, Girma T![]() 6 and Techane T7
6 and Techane T7

