Sodero G *1, Pane LC1, Sessa L1, Rotunno G1, Zampino G
*1, Pane LC1, Sessa L1, Rotunno G1, Zampino G 1,2, Rigante D
1,2, Rigante D 1,2 and Cipolla C1
1,2 and Cipolla C1
1Department of Life Sciences and Public Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
2Università Cattolica del Sacro Cuore, Rome, Italy
*Correspondence: Giorgio Sodero, Department of Life Sciences and Public Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Received on 12 January 2024; Accepted on 30 April 2024; Published on 02 May 2024
Copyright © 2024 Sodero G, et al. This is an open-access article and is distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction: Fetal alcohol syndrome (FAS) is associated with a positive maternal history of drinking during pregnancy and the presence of characteristic dysmorphic features due to alcohol teratogenic effect. No evidence is yet available regarding the use of growth hormone (GH) for children with FAS for whom GH deficiency has not been confirmed.
Case Presentation: We have highlighted an improvement in auxological parameters of a child with FAS and normal GH stimulation tests, using a GH dosage similar to that used in idiopathic GH deficit, without side effects.
Conclusion: GH therapy in children with FAS may lead to an improvement in growth rate and an increase in final height, although, this condition is not included in the specific recommendations for use of GH. Prolonged follow-up and prospective studies are needed to evaluate long-term efficacy and monitor any possible onset of side effects.
Keywords
growth hormone therapy, fetal alcohol syndrome, short stature, personalized medicine
Abbreviations
FAS: fetal alcohol syndrome; GH: growth hormone; SD: standard deviations; GHST: growth hormone stimulation test; GHD: growth hormone deficiency
Introduction
Short stature is defined as height less than 2 standard deviations (SD) [1]. A child with auxological parameters referred to as ‘short stature’ is routinely subjected to a growth hormone stimulation test (GHST) to distinguish between growth hormone deficiency (GHD) and other causes of short stature [2].
Short stature is also one of the clinical features of various syndromic conditions, genetic or acquired, such as fetal alcohol syndrome (FAS). This diagnosis is established by a positive maternal history of drinking alcohol during pregnancy and the presence of characteristic dysmorphic features due to the characteristic teratogenic effect of alcohol [3].
FAS is characterized by neurobehavioral disabilities (deficient global intellectual ability and abnormal behaviors with impaired achievement of adaptive skills) associated with typical facial and anthropometric anomalies (short palpebral fissures 10th percentile or less for age, thin vermilion border of the upper lip, smooth philtrum, and progressive growth retardation) [4].
Stopping maternal alcohol consumption during pregnancy is the most effective method of FAS prevention, while other therapies (prenatal administration of food antioxidant supplements, folic acid, choline, neuroactive peptides, neurotrophic growth factors) have limited efficacy and are poorly active for symptom control [3].
Despite the well-known relationship between FAS and short stature, no evidence is available regarding the use of growth hormone (GH) in these particular types of children in the absence of a confirmed GHD, and results regarding the efficacy of hormonal therapy in FAS are conflicting.
We report our experience with GH therapy in a 10-year-old patient with FAS being followed up at our center.
Case Report
Our patient is a boy adopted from Russia, born in 2010, who has lived in Italy since 2014, affected by FAS. He was periodically evaluated at the Pediatric Endocrinology Day Hospital of the Fondazione Policlinico Universitario A. Gemelli IRCCS to manage his condition, and, in particular, to assess growth velocity and his pubertal development.
No detailed information regarding pregnancy and birth was available, except for a history of alcohol abuse by this patient’s biological mother. The baby was born small for gestational age (weight 1.780 kg; length 42 cm; head circumference 30.1 cm) at T36 weeks of gestational age. He underwent full evaluation for the most common infectious diseases transmitted from mother to child (human immunodeficiency virus, hepatitis B, hepatitis C, toxoplasmosis, cytomegalovirus) and was found healthy.
The foster care environment selected by social services was found to be adequate. Child neuropsychiatric evaluations performed during periodic follow-up visits showed cognitive development at the lower normal level, assessed according to the Wechsler Intelligence Scale for Children (WISC).
In 2019, at 9 years, due to the detection of a slowdown in his growth rate (4 cm/year), the child was subjected to various lab tests (full blood count, antibodies for coeliac disease, thyroid function, and IGF-1); the hand-wrist X-ray showed a bone age comparable to patient’s chronological age, while brain magnetic resonance highlighted a simplification of the cerebral hemispheres in the absence of frank anatomical alterations of the hypothalamic-pituitary axis. The child also presented prepubertal characteristics, with gonadotropin and testosterone levels at the lower limits; the testicular volume was less than 4 ml.
As usual in children with an impaired growth rate, GHST was performed (with arginine), to exclude the presence of GHD, but resulted in normal (GH peak: 16.4 ng/ml). In June 2021, a reduced growth rate was confirmed (height -2.80 SD); in October 2021, another GHST was performed (with clonidine), giving another normal result (GH peak: 19.7 ng/ml).
During the endocrinological evaluation of June 2022, a low growth rate was confirmed with further loss of height SD (from -2.96 to -3.40 SD). Based on the growth rate slowdown and progressive reduction of height SD, it was therefore decided to start GH therapy, after obtaining a positive opinion from our regional commission as required by national guidelines, despite the absence of a documented GHD [5]. The dosage of GH used was similar to that used in the case of idiopathic GHD (0.028 mg/kg of body weight/day).
At the subsequent follow-up visit (January 2023), a modest increase in growth rate was highlighted (Figure 1) with significant improvement in height (132.1 cm) and height SD (from -3.40 to -3.16 SD). The child’s adoptive parents reported an excellent adherence to GH therapy and no side effects were reported. Blood tests were normal and no alterations in glycemia were found. His echocardiogram remained stable compared to previous controls.
In October 2023, a further increase in height was observed with an improvement in height SD (3.01 SD) with no side effects. No significant progression of secondary sexual characteristics was documented; therefore, the improvement in growth velocity and height was merely attributed to GH therapy. For this reason, the therapeutic indication was renewed, and the GH dosage was adjusted to the child’s weight without modifying the pro-kg dosage. The child is currently being followed up in our center, and GH therapy is still ongoing.
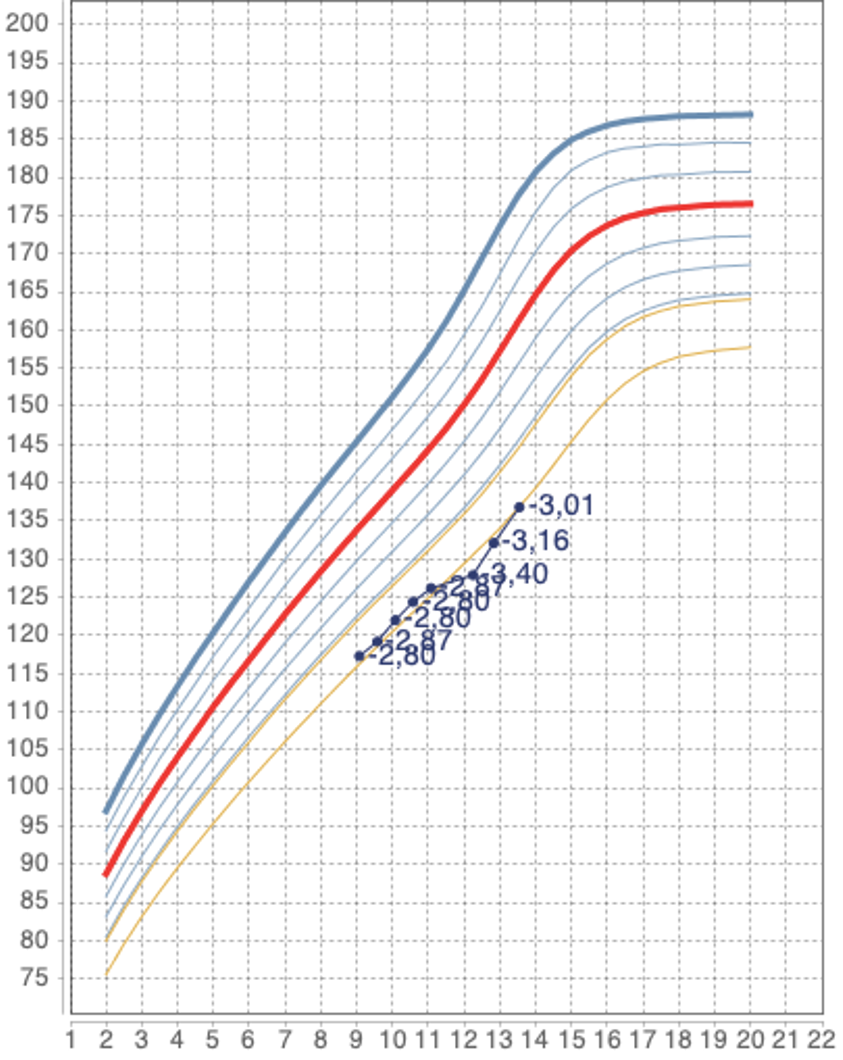 Figure 1: Height growth chart of the patient described in this report.
Figure 1: Height growth chart of the patient described in this report.
Discussion
GH therapy in patients with FAS is not standardized, and GH prescription is subordinated to the result of GHST. In this report, we have described our experience in a case of FAS receiving GH, despite normal GHST results on two occasions.
Short stature and slow growth rate are characteristics of children with FAS [3]. Due to multifactorial etiologies, alcohol is a well-established teratogen that can cause variable physical and behavioral effects on the fetus, and exposure to alcohol during pregnancy could increase IGF-I and IGF-II, also decreasing leptin in early childhood [6]. Baseline GH levels are decreased compared to children with normal height but are still within the normal range [7], and the response to classic GHSTs is normal in most cases. A study conducted on 5 children diagnosed with FAS showed normal or abnormal increased GH response (up to 150 ng/ml) [8], following classic GHSTs (arginine or hypoglycemia stimulation) with impaired growth rate and height below normal limits; in fact, these pseudopathological GH levels are not associated with a normal growth [9], and this may be explained by a partial peripheral resistance to GH similar to patients with Laron syndrome. Indeed, peripheral resistance to GH is one of the possible pathophysiological mechanisms of other conditions with stunted growth, including idiopathic short stature [10]. It is possible to hypothesize that increased GH levels might lead to receptor overstimulation, triggering the normal production of IGF-1 and improving the anthropometric parameters of these patients.
Through this report, we have highlighted an improvement in our patient’s auxological parameters using a GH dosage similar to that used in GHD (0.028 mg/kg/day). The prepubertal status of our patient, associated with his stable psychosocial condition and lack of previous infections, allowed us to hypothesize that the benefit of height gain should be attributed mainly to GH therapy.
Our experience is limited to a single patient, and data reported to FAS in the medical literature are conflicting. Therefore, a prolonged observation in a larger cohort of patients is necessary to evaluate both the efficacy and safety profile of GH in FAS patients.
GH therapy in children with FAS may lead to an improvement in growth rate and an increase in final height, although, this condition is not included in the specific recommendations for use of GH. Prolonged follow-up and prospective studies are needed to evaluate long-term efficacy and monitor any possible onset of side effects.
Ethics Approval and Consent to Participate
The growth hormone therapy has been approved by the regional commission of the Lazio region, Italy, in accordance with national guidelines.
Consent for Publication
The parents of the patient have consented to the publication of their child’s information and have signed an informed consent.
Conflicts of Interest
The authors declare that they have no disclosures in relationship with the subject of this report.
References
- Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990-993.
- Sodero G, Mariani F, Caprarelli M, et al. Growth hormone responses during arginine and clonidine stimulation test: Correlations with patients’ auxological and metabolic parameters in a single centre study. Growth Horm IGF Res. 2023;68:101522.
- Gupta KK, Gupta VK, Shirasaka T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin Exp Res. 2016;40(8):1594-602.
- Denny L, Coles S, Blitz R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am Fam Physician. 2017;96(8):515-22.
- Agenzia Italiana Del Farmaco. Nota 39.
- Aros S, Mills JL, Iñiguez G, et al. Effects of prenatal ethanol exposure on postnatal growth and the insulin-like growth factor axis. Horm Res Paediatr. 2011;75(3):166-73.
- Hellström A, Jansson C, Boguszewski M, et al. Growth hormone status in six children with fetal alcohol syndrome. Acta Paediatr. 1996;85(12):1456-462.
- Tze WJ, Friesen HG, MacLeod PM. Growth hormone response in fetal alcohol syndrome. Arch Dis Child. 1976;51(9):703-06.
- Castells S, Mark E, Abaci F, et al. Growth retardation in fetal alcohol syndrome. Unresponsiveness to growth-promoting hormones. Dev Pharmacol Ther. 1981;3(4):232-41.
- Savage MO, Storr HL. GH Resistance Is a Component of Idiopathic Short Stature: Implications for rhGH Therapy. Front Endocrinol (Lausanne). 2021;12:781044.
![]() *1, Pane LC1, Sessa L1, Rotunno G1, Zampino G
*1, Pane LC1, Sessa L1, Rotunno G1, Zampino G![]() 1,2, Rigante D
1,2, Rigante D![]() 1,2 and Cipolla C1
1,2 and Cipolla C1
