Glycogenic hepatopathy (GH) refers to a relatively rare complication of diabetes mellitus (DM) type 1 which constitutes of reversible accumulation of excess hepatic glycogen, usually manifesting in the form of liver enzyme elevation along with hepatomegaly. The occurrence of the disorder is most commonly related to inadequate control of blood sugars. Herein, we report upon a case of an 18-year-old girl presenting with severe transaminase elevations owing to poor metabolic control, as well as the progression of her enzymes and general condition during and after her hospitalization.
diabetes mellitus type 1, glycogenic hepatopathy, liver enzymes, transaminases, blood glucose control, hepatomegaly
GH: glycogenic hepatopathy; DM: diabetes mellitus; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; ED: emergency department
Glycogenic hepatopathy (GH) is a clinical syndrome constituting pathologic added hepatic glycogen that is usually associated with diabetes mellitus (DM) in a state of inadequate control. The condition is characterized by a disordered production and degradation of glycogen, leading to excess hepatic glycogen accumulation and transaminase leakage outside of hepatocytes [1]. The disorder has additionally been described as a complication of other clinical entities, first of all, DM type 2 [2], but also anorexia nervosa, dumping syndrome, and short-term intense steroid therapy [3–6].
Both types of DM can potentially affect all systems of the body, including the liver. Of note, the reverse state can also appear, where DM occurs as a complication in cirrhotic patients [7]. A combination of increased inflammation, intense oxidative stress, and altered macronutrient metabolism as the disease progresses can result in non-alcoholic fatty liver disease (NAFLD) and eventually non-alcoholic steatohepatitis (NASH), hepatic cirrhosis and, progressively, hepatocellular carcinomas [8]. Although NAFLD and NASH occur with increased frequency among type 2 DM patients, with a respective prevalence of 55.5% and 37.3% in adults [9], the burden is high in adult type 1 DM patients as well, with a prevalence of 22.0%, according to recent systematic reviews and meta-analyses [10].
In the current paper, we report an uncommon case of a teenage girl presenting to the Emergency Department (ED) with non-specific complaints to be diagnosed with hepatomegaly and altered hepatic biochemistry owing to inadequate control over her diet and, as a result, over her blood glucose. Furthermore, the progression of her liver function tests during her hospitalization, as well as during the follow-up phase, are presented and discussed.
An 18-year-old female with a personal medical history of type 1 DM for the last 7 years presented at the ED of our hospital with complaints of bloating and general malaise. She was treated with an antidiabetic regimen of 18 International Units (IU) of insulin glargine daily, as well as insulin aspart before each of her meals on doses she controlled herself based on the macronutrient content of her meals. Other than DM, her medical history was insignificant and she denied alcohol, tobacco, and any other drug use. When further questioned, the patient also complained of amenorrhea for the last year. Upon arrival at the ED, her vital signs were stable, with a blood pressure of 130/76 mmHg, respiratory rate of 16 per min for an oxygen saturation of 99% on air, a regular pulse of 85 beats per min, and a temperature of 36.3°C. Clinical examination was normal in its entirety, with chest, abdominal and neurologic examination presenting no pathologic findings. Her weight was 58 kg, and her height was 1.64 m for a body mass index (BMI) of 21.6 kg/m2. A complete blood count and full biochemical and immunological profile were requested, with results being depicted in the table (Table 1). The patient’s blood glucose upon presentation at the ED was 252 mg/dL. Regarding hepatic function, liver and cholestatic enzymes presented abnormalities, while INR was within the normal range. Viral profiling showed no evidence of hepatitis or mononucleosis infection. A liver and biliary tract ultrasound was obtained, which revealed a slightly enlarged liver and increased parenchymal echogenicity, indicative of low-degree fatty liver alterations, without apparent tumors or masses. The biliary tract was normal.
| Examination | Patient value | Laboratory reference range |
| Complete blood count |
| White blood cells (K/μL) | 9.7 | 4.0–10.8 |
| Red blood cells (M/μL) | 5.0 | 4.5–6.1 |
| Haemoglobin (g/dL) | 15.2 | 13.5–17.9 |
| Haematocrit (%) | 47.1 | 40.0–52.0 |
| Platelets | 461 | 150–440 |
| Biochemical profile |
| Potassium (mmol/L) | 4.40 | 3.60–5.00 |
| Sodium (mmol/L) | 139 | 135–150 |
| Glucose (mg/dL) | 252 | 70–110 |
| Urea (mg/dL) | 33.0 | 20.0–50.0 |
| Calcium (mg/dL) | 10.50 | 8.50–10.10 |
| Lactate dehydrogenase (U/L) | 365 | 120–246 |
| Creatine phosphokinase (U/L) | 38 | 30–170 |
| Alkaline phosphatase (U/L) | 160 | 38–126 |
| γ-glutamyl transferase (U/L) | 99 | 8.0–78.0 |
| Aspartate aminotransferase (U/L) | 333 | 15–59 |
| Alanine aminotransferase (U/L) | 187 | 10–72 |
| INR | 0.89 | 0.85–1.15 |
| Thyroid-stimulating hormone (mIU/L) | 1.8 | 0.30–5.00 |
| HBA1c (%) | 12.4 | 4.0–6.0 |
| Folic acid (ng/mL) | 11.0 | 3.0–14.2 |
| Ferritin (mg/L) | 41.2 | 10.0–150.0 |
| C-reactive protein (mg/L) | 3.3 | 0.0–5.0 |
| Procalcitonin (ng/mL) | 0.02 | < 0.10 |
| Immunological profile |
| IgG (g/L) | 11 | 7–17 |
| IgM (g/L) | 1.41 | 0.40–280 |
| IgA (g/L) | 3.1 | 0.7–4.0 |
| IgE (g/L) | 17 | 0–100 |
| Ceruloplasmin (g/L) | 0.25 | 0.20–0.55 |
| β₂-microglobulin (mg/L) | 1.7 | 0.7–2.0 |
| α1-antitrypsin (g/L) | 1.2 | 0.9–2.5 |
| Haptoglobin (g/L) | 1.4 | 0.3–2.0 |
| Rheumatoid factor | 9 | 0–20 |
| ED viral profiling |
| HAV IgM antibodies | Negative | – |
| HAV IgG antibodies | Positive | – |
| HBsAg | Negative | – |
| HBeAg | Negative | – |
| Anti-HBe | Negative | – |
| Anti-HBc | Negative | – |
| Anti-HBs | Negative | – |
| HCV antibodies | Negative | – |
| HIV antigen-antibodies | Negative | – |
| MONO test | Negative | – |
Table 1: Complete blood count and biochemical examination at the ED.
The patient was admitted to the Internal Medicine Unit of the hospital, where she underwent a full panel of investigations for her altered liver function. Specifically, antibodies against hepatitis D and E (negative), antibodies against herpes zoster virus one and two (negative IgM and IgG antibodies), antibodies against varicella zoster virus (negative IgM, positive IgG antibodies), antibodies against Coxiella burnetii (negative IgM and IgG antibodies), antibodies against Epstein-Barr virus (negative IgM, positive IgG antibodies), antibodies against cytomegalovirus (negative IgM and IgG antibodies), anti-nuclear antibodies (negative), anti-smooth muscle antibodies (negative), anti-mitochondrial antibodies (negative), anti-liver-kidney microsomal antibodies (negative), anti-tissue-transglutaminase IgA antibodies (negative) were checked. As a next step, a computed tomography (CT) scan of the upper abdomen was performed, which showed only mild liver enlargement without further pathology, confirming the findings of the ED ultrasound. A liver biopsy was performed, revealing micro- and macrovesicular steatosis in an area covering approximately 30% of the liver parenchyma, but without apparent sources of inflammation or pericellular fibrosis.
The patient was placed on a closely monitored diet along with a long- and short-acting insulin regimen to ensure better metabolic control. However, due to occasional misconduct, namely skipping mealtimes throughout the day, liver enzyme values showed rises and falls throughout the patient’s hospitalization. After a dietologist consult to ensure palatability and ease in meal timing, the patient was able to commit to a diet that allowed for adequate glycemic control and, as a result, liver enzyme values dropped significantly towards the end of her hospitalization and were within normal limits on her follow-up visit a few days later (Figure 1). The progression of hepatic enzyme values can be found in the table (Table 2). During her hospitalization, the patient presented no further clinical complaints and she was able to return home safely on the 18th day after her hospital admission.
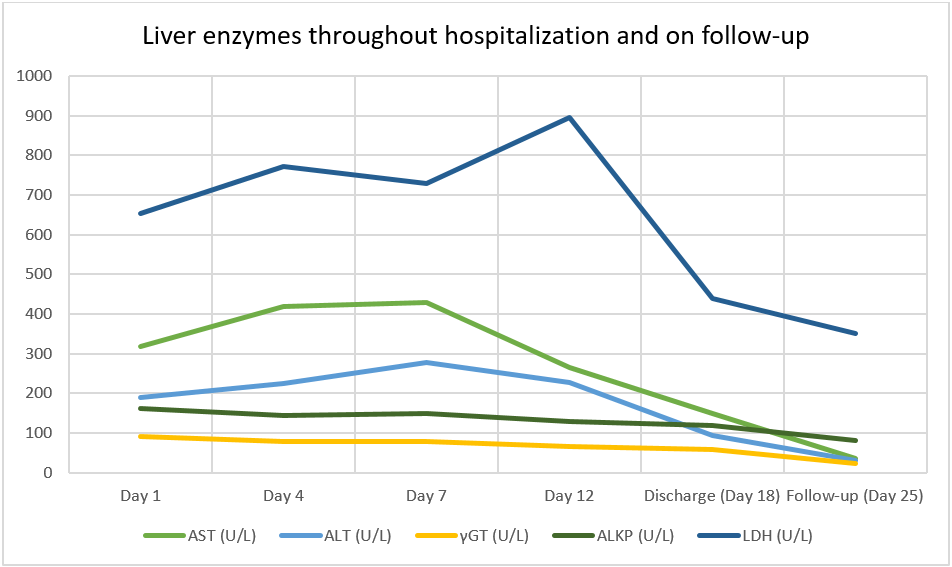 Figure 1: Liver enzyme values throughout the hospitalization and on follow-up
Figure 1: Liver enzyme values throughout the hospitalization and on follow-up
| Parameter | Day 1 | Day 4 | Day 7 | Day 12 | Discharge (Day 18) | Follow-up (Day 25) |
| AST (U/L) | 318 | 419 | 428 | 265 | 150 | 35 |
| ALT (U/L) | 190 | 225 | 279 | 228 | 94 | 30 |
| γGT (U/L) | 90 | 78 | 79 | 66 | 58 | 22 |
| ALP (U/L) | 162 | 145 | 149 | 128 | 119 | 82 |
| LDH (U/L) | 653 | 771 | 729 | 897 | 440 | 352 |
| Fasting Glu 8 AM (mg/dL) | 154 | 348 | 148 | 159 | 101 | 98 |
Table 2: Progression of liver enzyme values throughout the hospitalization and on follow-up. AST: aspartate aminotransferase; ALT: alanine aminotransferase; γGT: γ-glutamyl transferase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; Glu: glucose.
In the present study, we describe a case of a teenage girl with type 1 DM and the rare complication of GH. Owing to poor glycemic control, the patient developed hepatic complications, namely increased liver size along with elevated liver enzymes, with the latter subsiding after her discharge, when optimal blood sugar control had been achieved through diet and lifestyle modifications. Our differential diagnosis included NAFLD and NASH, conditions that could be ruled out based on the radiology and biopsy findings.
Originally described by Mauriac, who also baptized the syndrome, glycogenic hepatopathy represents a nowadays rare complication of type 1 DM, which consists of stunted growth and puberty, hepatomegaly, and moon faces, owing to poor blood glucose control. Without treatment, patients can eventually develop severe hepatomegaly, low blood glucose, consecutive hyperlactatemia, and hyperlipidemia [11]. Newer insulin formulations enabled better round-the-clock insulin delivery and glycemic control, leading to a dramatic decrease in poorly controlled diabetics, at least in the developed world, thus almost eliminating the syndrome. However, reports of its cardinal features among young people with newly diagnosed type 1 DM still appear in the medical literature [12, 13]. Although the exact etiopathogenetic mechanism has not been fully studied, it is believed that fluctuations in blood glucose values with subsequent alterations in insulin production and delivery lead to increased excessive glycogen storage within the liver. At the same time, genetic abnormalities in the coding genes for glycogen synthase and glucose 6-phosphatase have been described, which may lead to disordered glycogen metabolism [14].
After the exclusion of other clinically relevant disorders, the diagnosis can be made through liver biopsy, which reveals large liver cells with excess depositions of glycogen, without signs of inflammation or steatosis. However, other case reports have described cases whose liver biopsies included findings of hepatic fibrosis [15, 16]. In any case, recognition of the syndrome leads to the realization of the need for optimal glycemic control, which in turn reverses hepatic involvement.
GH represents a rather uncommon, usually benign hepatic complication of inadequately controlled type 1 DM, presenting with liver enzyme elevations and acute hepatomegaly. The condition is commonly associated not only with acute hepatic enzyme derailments but also micro- and macrovesicular steatosis on the liver biopsy. Upon recognition of the syndrome and optimal glycemic control, the clinical implications of the syndrome can rapidly reverse.

