A gastric adenoma is designated as a benign, circumscribed, gastric epithelial lesion. Gastric adenoma emerges as a component of gastric polyp although flat and depressed mucosal lesions may concur. Neoplasm is associated with microsatellite instability and genomic mutations of the APC gene. A gastric adenoma is contemplated to be analogous to colonic dysplasia-associated tumefaction emerging in concurrence with chronic inflammatory bowel disease. Akin to gastric adenocarcinoma, the incidence of gastric adenoma is enhanced with increasing age. A male predominance is observed with a male-to-female proportion of ~3:1 [1, 2].
A gastric adenoma is associated with conditions such as atrophic gastritis with metaplasia or autoimmune gastritis, intestinal metaplasia, or familial adenomatous polyposis. Exceptionally, gastric adenoma emerges in concurrence with reactive or chemical gastropathy or may arise following gastric antrectomy [1, 2].
Gastric adenoma generally manifests as a solitary, sessile, or pedunculated, polypoid lesion of up to 4-centimeter magnitude. Flattened lesions depicting circumscribed foci of epithelial dysplasia are denominated as ‘flat adenoma’. Exceptionally, depressed mucosal lesions may be discerned. A gastric adenoma may configure as a villous, tubular or tubulovillous lesion [1, 2].
A gastric adenoma may demonstrate polypoid projections layered with pseudo-stratified dysplastic epithelium superimposed upon glandular articulations with cystic dilatation devoid of epithelial dysplasia and disseminated endocrine cells or Paneth cells. Epithelial cells display nuclear anomalies and frequent mitotic activity. Gastric atrophy and intestinal metaplasia may concur [1, 2].
Gastric adenoma with high-grade dysplasia exhibits cribriform, irregular, branched, or crowded glands with budding. Cytological atypia is significant [1, 2].
Gastric adenoma exemplifies distinctive subtypes as
- intestinal type is a commonly discerned variant that configures tubular, villous, or mixed adenomas with a specific orientation of the superficial epithelial surface. Besides, flat or depressed adenomas may appear. The intestinal type of gastric adenoma exhibits focal goblet cells and Paneth cells. Epithelial cell nuclei appear elongated and hyperchromatic. Foci of epithelial dysplasia are confined and accentuated towards the superficial surface. Atrophic gastritis or gastritis due to Helicobacter pylori infection and intestinal metaplasia may concur. However, the intestinal type of gastric adenoma is nonconcurrent with familial adenomatous polyposis [1, 2]. Intestinal gastric adenoma exhibits an enhanced incidence of high-grade dysplasia or invasive gastric carcinoma. An isolated focus of gastric adenocarcinoma may be discerned [1,2].
- gastric type of adenoma is comprehensively layered by mucin-secreting gastric cells, as discerned with Periodic-Acid Schiff stain or Alcian blue stain. Gastric type adenoma is predominantly solitary and may be discerned within diverse gastric segments. The adjacent gastric mucosa is unremarkable and coexistent gastric carcinoma is absent [1, 2].
- foveolar type of gastric adenoma is layered with a gastric variety of foveolar cells with apical mucin vacuoles. Mucin caps can be stained with Periodic-Acid Schiff stain with diastase resistance and appear non-reactive to Alcian blue. Goblet cells and Paneth cells are absent. Epithelial cells are imbued with elongated, hyperchromatic nuclei [1, 2]. The foveolar type of gastric adenoma is frequently associated with familial adenomatous polyposis. Generally, an amalgamation of ~ three adenomas, confined to the gastric fundus, is observed. Association with chronic gastritis or intestinal metaplasia is exceptional. High-grade dysplasia and gastric carcinoma are infrequently discerned [1, 2].
- pyloric gland type of gastric adenoma is preponderantly situated within the gastric fundus and is constituted of intensely aggregated pyloric gland tubules interspersed with tubules frequently demonstrating cystic dilatation. However, lesions may be disseminated evenly within diverse gastric regions. Additionally, extra-gastric sites such as the duodenum, bile duct, or gall bladder appear incriminated, demonstrating patches of the gastric heterotopic epithelium [1, 2]. Pyloric gland type gastric adenoma is entirely configured of aforesaid constituents, in contrast to the surface orientation of cells encountered in intestinal type and foveolar type gastric adenoma. Neoplastic cells appear non-reactive to Alcian blue and Periodic-Acid Schiff stain with diastase resistance. Goblet cells or apical mucinous cap appear absent. Pyloric gland type gastric adenoma is comprised of abridged, tubular cells frequently imbued with ground glass cytoplasm and miniature, spherical, basal nuclei. Cytological features of dysplasia are absent although few lesions may depict mild dysplasia. Nuclei appear minimally elongated, pseudostratified, and hyperchromatic [1, 2]. Lesions with high-grade dysplasia demonstrate complex, cribriform pyloric glands delineating enlarged, spherical nuclei, loss of nuclear polarity, and enlarged nucleoli. Foci of intra-mucosal or invasive gastric carcinoma may be discerned [1, 2]. Pyloric gland type gastric adenoma is associated with atrophic gastritis and intestinal metaplasia. Familial adenomatous polyposis or Lynch syndrome may concur [1, 2].
- oxyntic gland polyp or gastric adenoma demonstrates aggregates, clusters, or glands and cords comprised of oxyntic mucosa. The lesion is centered deep within the mucosa of the gastric fundus or cardia [1, 2]. Oxyntic gland adenoma is layered with mucus-secreting chief cells and parietal cells discerned within the neck. Cellular clusters are intermingled with smooth muscle fibers. Neoplasm is devoid of significant cellular and nuclear pleomorphism, mitotic activity, necrosis, or vascular invasion. Oxyntic cell adenoma appears incapable of mounting a desmoplastic response. Tumor reoccurrence or distant metastases is absent [1, 2]. Occasionally, serrated adenomas appear confined to gastric cardia and depict an enhanced incidence of gastric carcinoma [1, 2].
- indeterminate subtype of gastric adenoma may lack appropriate characterization on account of lack of mucin production [1, 2].
A gastric adenoma is immune-reactive to CEA and p53 [3, 4]. A gastric adenoma is a precursor lesion of gastric adenocarcinoma. Flat, depressed, or enlarged adenomas > 2-centimeter magnitude may demonstrate foci of gastric carcinoma at initial disease discernment or may be associated with the enhanced possible occurrence of gastric carcinoma. Additionally, gastric carcinoma may be delineated within adjacent mucosa [3, 4].
In contrast to colonic adenoma, the gastric adenoma may frequently progress to gastric carcinoma. Intestinal type of gastric adenoma is associated with a significant probability of the emergence of gastric carcinoma [3, 4].
A gastric adenoma is appropriately alleviated with comprehensive surgical eradication of the lesion [3, 4].Cogent tissue sampling of non-polypoid adjacent gastric mucosa, especially within gastric antrum and corpus is recommended. Endoscopic surveillance adopted for discernment of gastric adenoma appears beneficial. Eradication of Helicobacter pylori infection is recommended [3, 4].
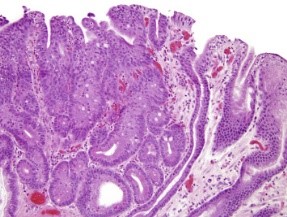
Figure 1: Gastric adenoma depicting glandular articulations lined
with pseudostratified mucus secreting columnar epithelium with focal
dysplasia, hyperchromatic nuclei, and cellular pleomorphism [5].
![Figure 2: Gastric adenoma delineating villous and glandular articulations lined with pseudostratified, mucus secreting columnar epithelium with delicate, inflamed, intervening lamina propria and red cell extravasation [6].](https://seriesscience.com/wp-content/uploads/2022/10/Figure-2-300x225.png)
Figure 2: Gastric adenoma delineating villous and glandular articulations lined with
pseudostratified, mucus secreting columnar epithelium with delicate, inflamed,
intervening lamina propria and red cell extravasation [6].


![Figure 2: Gastric adenoma delineating villous and glandular articulations lined with pseudostratified, mucus secreting columnar epithelium with delicate, inflamed, intervening lamina propria and red cell extravasation [6].](https://seriesscience.com/wp-content/uploads/2022/10/Figure-2-300x225.png)