Objective: Osteoporotic fractures are a major public health problem worldwide. Effective therapies have been available since 1995, with bisphosphonates (BPs) as the most used first-line drugs. Zoledronic acid and denosumab (Dmab) became available soon thereafter. However, both BPs and Dmab, but not other osteoporosis therapies, are associated with rare occurrences of osteonecrosis of the jaw (ONJ) and atypical femur fracture (AFF), the two most common major barriers either for initiating or maintaining effective anti-fracture therapies. In this pilot study, we aimed to determine dentists’ perceptions and practice patterns to mitigate ONJ.
Methods: After approval from the Institutional Review Board, we e-mailed an online 7-point survey questionnaire to licensed dental practitioners, the members of the Michigan Dental Association (MDA), to ascertain the range of approaches dentists would take to treat patients who were using osteoporosis medications.
Results: Members of the MDA were surveyed with a series of questions, and 40 responses were received. Respondents were 50% women, and 28 worked in suburban areas, 8 in urban areas, and 4 in rural areas. 48% of the dentists have been practicing for > 20 years, 32% for 5–20 years, and 20% for < 5 years. When asked about the interruption of osteoporosis medications for low-risk dental procedures, such as cavities, 68% never stopped therapy, 17% stopped in certain scenarios, and 15% reported stopping after a discussion with the patient’s provider. When asked about the interruption of osteoporosis medications for high-risk dental procedures, such as tooth extractions, 15% would not interrupt therapy, 23% would interrupt in certain scenarios, 7% would always interrupt therapy, and 55% would interrupt therapy after discussion with the patient’s provider.
Conclusion: Despite the extreme rarity of ONJ, no specific guidelines exist from the American Dental Association (ADA), and dentists’ perspectives on mitigating ONJ are quite varied. Our study suggests that many dentists feel that osteoporosis therapy is a major barrier to safe dental practice and indicates an urgent need for education of our dentist colleagues on the rationale and validity of interrupting osteoporosis therapies.
osteoporosis, ONJ, MRONJ, bisphosphonates, denosumab
ONJ: osteonecrosis of the jaw; AFF: atypical femur fracture; MDA: Michigan Dental Association; ADA: American Dental Association; BPs: bisphosphonates; Dmab: denosumab; MRONJ: medication-related osteonecrosis of the jaw; AAOMS: American Association of Oral and Maxillofacial Surgeons; FDA: Food and Drug Administration; FLEX: Fracture Intervention Trial Long-Term Extension
Osteoporosis is a significant public health problem affecting over 200 million people globally, with 1 in 5 men and 1 in 3 women sustaining an osteoporotic fracture within their lifetime [1]. One-year mortality after a hip fracture can range from 12–37%, which underscores the importance of treatment with the best available effective therapies [2]. Several medications are used for the prevention and treatment of osteoporosis, the most common being bisphosphonates (BPs) and denosumab (Dmab) [3, 4]. These antiresorptive agents show a significant reduction in vertebral and hip fractures by reducing bone turnover [5]. Despite the vast benefits associated with OP treatment in reducing morbidity and mortality, concerns have risen regarding the potential impact of BPs and Dmab, but not other anti-fracture medications, on dental procedures due to their known effect on bone metabolism and the pathogenesis of osteonecrosis of the jaw (ONJ) and atypical femur fracture (AFF) [6–9].
Medication-related osteonecrosis of the jaw (MRONJ) is a severe adverse drug reaction consisting of progressive bone destruction in the maxillofacial region, mostly involving the alveolar bone of the maxilla and mandible, in a patient not exposed to radiation [6]. Several medications are implicated in the development of MRONJ, but the two mains are BPs and Dmab [10]. MRONJ associated with OP therapy was first reported in 2004, with 63 patients noted over a 2-year period [11]. However, ONJ has been reported with various interventions as well as infections and radiation long before BPs became available for clinical use [12–14], and thus, ONJ is not specific to the use of BPs or Dmab. The pathophysiology of ONJ and AFF remains poorly understood. Proposed mechanisms include severe suppression of bone turnover, altered bone material properties, constant microtrauma, soft tissue BP toxicity, and underlying inflammation or infection [8, 15–18]. Patients at increased risk of MRONJ include those receiving antiresorptive agents at higher dosages for cancer-related indications, those receiving BPs and Dmab for more than 2 years, and those with periodontitis or dentures. Oral surgery is one of the greatest risk factors for MRONJ, with 52–61% of patients reporting tooth extraction as a precipitating event [19].
The risk of MRONJ among patients treated with BP is estimated to be 0.02–0.05%, which is interestingly comparable to patients receiving a placebo [20, 21]. The risk of ONJ in patients taking Dmab, although numerically very low, is 10-fold higher than in patients taking BPs at 0.3% after 10 years of exposure [22]. Regardless of the indication for therapy, the duration of antiresorptive therapy is a major risk factor for developing MRONJ as it is for AFF [17]. Current estimates for MRONJ after dentoalveolar procedures range between 0–0.15% risk in patients on BP and up to 1% for patients exposed to Dmab [23]. In this pilot study, we aimed to determine dentists’ perceptions and practice patterns on the interruption of OP medications. By shedding light on their attitudes, knowledge, and practices, this survey study aims to provide valuable insights into the current state of dental care coordination.
After obtaining approval from the Henry Ford Institutional Review Board, we conducted a descriptive online survey study to assess dentists’ perceptions regarding the interruption of BPs and Dmab for dental procedures. The target population for this study consisted of registered members of the Michigan Dental Association (MDA) who were all licensed dental professionals.
The survey consisted of seven questions designed to gather information on the demographic characteristics of the participants and the range of approaches dentists would take to treat patients using osteoporosis medications. The questionnaire was developed based on the review of relevant literature and expert input.
The survey was sent out at two different time points, two weeks apart, between March 2023 and April 2023. The goal was to ascertain the range of approaches dental practitioners would take to treat patients who were using BPs or Dmab at the time of treatment. We used descriptive analysis to summarize survey data. The study ensured participant confidentiality and anonymity during the data collection process.
Among the 40 respondents of the MDA, 50% identified as women, and their practice locations were distributed as follows: 28 in suburban areas, 8 in urban areas, and 4 in rural areas. About half (48%) of the dentists had been practicing for more than 20 years, 32% for 5–20 years, and 20% for less than 5 years (Table 1).
| Measure | Item | Count | Percentage (%) |
| Gender | Male | 20 | 50 |
| Female | 20 | 50 |
| Seen MRONJ before? | Yes | 25 | 62.5 |
| No | 15 | 37.5 |
| Years practicing dentistry | < 5 years | 8 | 20 |
| 5–10 years | 7 | 17.5 |
| 10–20 years | 6 | 15 |
| > 20 years | 19 | 47.5 |
| Practice location | Rural | 4 | 10 |
| Suburb | 28 | 70 |
| Urban | 8 | 20 |
Table 1: Demographics of survey participants.
About 58% of the MDA providers reported that they routinely ask about the use of either BPs or Dmab before any dental procedure, while 28% asked some of the time, and the remainder asked rarely (Figure 1). Approximately 63% of MDA providers reported encountering cases of MRONJ in their practice, while the rest stated they had not come across any cases.
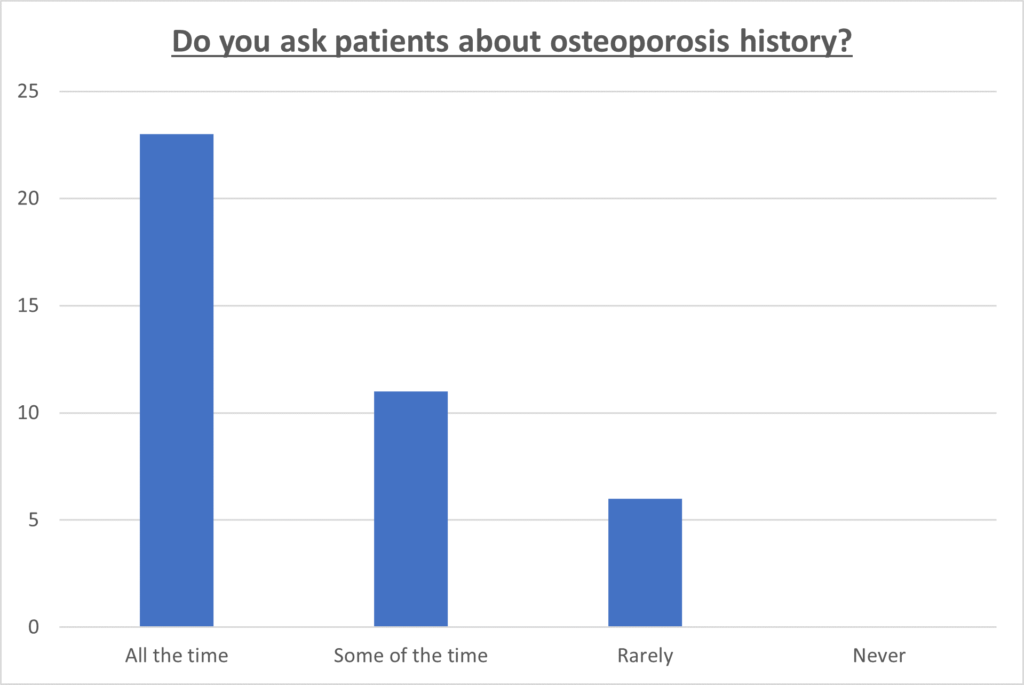 Figure 1: Dental practitioners’ acknowledgment of osteoporosis history in patients.
Figure 1: Dental practitioners’ acknowledgment of osteoporosis history in patients.
Concerning the interruption of osteoporosis medications for low-risk dental procedures like cavity treatment, 68% of the MDA providers stated that they never stopped therapy. 17% reported stopping medication in certain scenarios, while 15% reported stopping therapy after discussing the matter with the patient’s healthcare provider. Regarding the interruption of osteoporosis medications for high-risk dental procedures such as tooth extractions, 15% of the providers indicated that they would not interrupt therapy under any circumstances. 23% reported interrupting therapy in certain scenarios, 7% stated they would always interrupt therapy, and 55% reported interrupting therapy after discussing the matter with the patient’s healthcare provider (Figure 2).
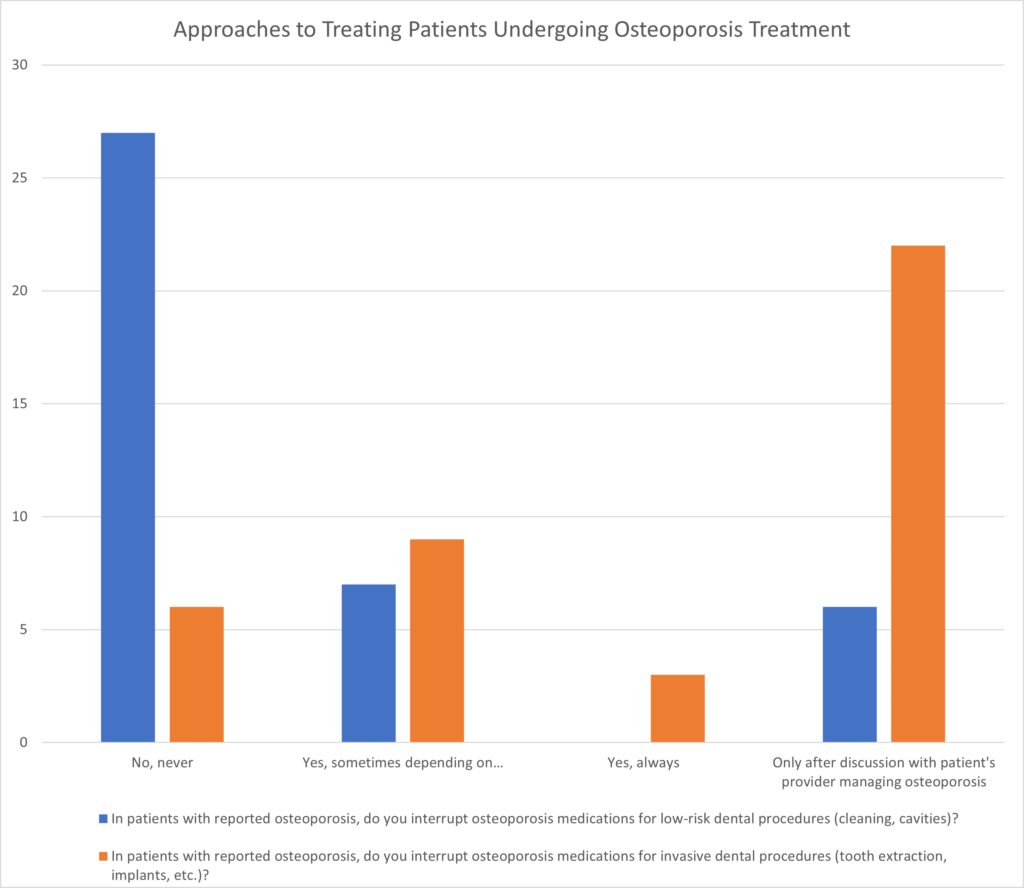 Figure 2: Approaches to treating patients undergoing osteoporosis treatment.
Figure 2: Approaches to treating patients undergoing osteoporosis treatment.
The concept of a drug holiday is not unique for BPs as it is used for patients with Parkinsonism and depression. A drug holiday for patients receiving BPs or Dmab, who require invasive dental procedures, remains a controversial subject, with limited data to support its practice. Even among dental associations, guidelines regarding the interruption of osteoporosis medications for dental procedures are not uniform. In 2009, the American Association of Oral and Maxillofacial Surgeons (AAOMS) position paper recommended a drug holiday of 3 months before and after invasive dental surgery for patients exposed to BP therapy for over 4 years [24]. However, in 2011, the U.S. Food and Drug Administration (FDA) stated that there was insufficient data available to guide decisions on the initiation or duration of a drug holiday [25].
The American Dental Association (ADA) suggests that the discontinuation of antiresorptive therapy should be a medical decision based primarily on the risk of experiencing skeletally related events, such as fractures, rather than the potential risk of developing MRONJ [26]. The ADA further emphasizes that no oral or maxillofacial surgical procedure is strictly contraindicated due to antiresorptive therapy [26].
Guidelines do recommend a focus on preventing MRONJ through a multidisciplinary approach. Before initiating antiresorptive therapy, patients should undergo a comprehensive oral examination, receive oral hygiene instructions, and be educated about the risk of developing MRONJ [26]. Any necessary dental treatment, especially invasive procedures like dental extractions, should be completed before initiating antiresorptive therapy [27]. Khosla et al. [28] reported on the current crisis in the treatment of OP in the Journal of Bone and Mineral Research. This article noted the concerns the media reported on rare side effects of OP therapy leading to a dramatic decrease in OP treatment. Due to growing concerns in public, there was a 50% reduction in OP therapy use from 2008 to 2012. This crisis sheds light on the need for information clarification for both providers and patients [28].
Our survey study sheds light on the lack of uniform guidelines for dental procedures, which subsequently leads to varied practices among dentists. As the population continues to age, the number of patients receiving OP therapies will increase. To mitigate the risk of MRONJ, it is crucial to foster a collaborative approach among dentists and healthcare providers who prescribe antiresorptive therapies.
Furthermore, abrupt cessation of BP would not mitigate the risk of MRONJ, as commonly assumed, since these medications exhibit a sustained antiresorptive effect even after discontinuation. The Fracture Intervention Trial Long-Term Extension (FLEX) demonstrated that individuals who stopped alendronate after five years continued to experience a reduction in fracture risk, emphasizing the enduring impact of BP on bone health [29]. The concept of a three-month drug holiday overlooks the nuanced interplay between BP pharmacokinetics and their therapeutic benefits. In light of the accumulating evidence supporting the sustained effects of BP, a more evidence-based approach to drug holiday recommendations for dental procedures is warranted. Similarly, abrupt cessation of Dmab may increase the risk of rebound-associated vertebral fractures [30], although this is an area of continued debate [31].
Our study has limitations that should be acknowledged in interpreting the findings. First, the study focused exclusively on registered members of the MDA, potentially limiting the generalizability of our results to dental professionals outside this specific organization’s demographics. Second, the reliance on self-reported data introduces the potential for response bias as participants who chose to respond may differ systematically from those who chose not to participate. Third, the brevity of the survey, consisting of only seven questions, may have limited the depth of information collected, providing only a more superficial understanding of dentists’ knowledge and perceptions.
In conclusion, the controversy surrounding drug holidays for osteoporosis medications in the context of dental procedures reflects the absence of uniform guidelines and varying practices among dentists. A multidisciplinary approach, including collaboration between dentists and providers prescribing antiresorptive therapies, is necessary to reduce the risk of MRONJ. Standardized guidelines that consider the balance between skeletal events and MRONJ risk are required to ensure consistent and evidence-based care for patients receiving osteoporosis treatment who require dental procedures. Further research and collaboration in this field are essential to optimize patient outcomes and oral health in this growing patient population.
The authors have no conflicts to disclose.


