A novel coronavirus (SARS-CoV-2) infection as named COVID-19, which firstly begun in Wuhan, China has entered the world literature by December 2019. Besides being asymptomatic in general, severe pneumonia and fatality ratios were also realized to be very high, and the infection spread all over the world in a small period of time [1]. World Health Organization (WHO) declared the infection as COVID-19 pandemic on March 11, 2020. By the day (26.05.2020); global death number is over 350.000 and there are nearly 5.9 million confirmed cases [2].
Diabetes, as a widespread chronic disease which affects the whole organism, is considered as an epidemic of the 21st century by patient number increase of 108 million to 422 million from 1980 to 2014 [3]. Also, this number is predicted to be 642 million by the year 2040 [4]. As a chronic disease, diabetes causes various acute and chronic complications [5]. Besides, it has been shown to increase infection and infectious complication susceptibility in patients [6–8]. Comorbidities as; diabetes, chronic heart diseases, chronic pulmonary diseases and smoking are considered as important risk factors in acute and chronic outcomes of respiratory system diseases including pneumonia [9]. Among the other comorbidities, diabetes had been detected to be a negative factor in disease process of the two recent coronavirus epidemics, SARS, and MERS [7, 10]. Likewise, mentioned comorbidities have been shown to be important risk factors for complications and fatality in COVID-19 pandemic [10, 11].
By this point, evaluation of COVID-19 and diabetes relation will be important in COVID-19 road map. For this purpose, a systematic review to determine, whether there exists a relation between COVID-19 and diabetes among other comorbidities, has been planned in this study.
This systematic review has been done to analyze the current literature about COVID-19 and diabetes relation. For the review ‘The Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ (PRISMA) guidelines were used.
Eligibility criteria for research selection were determined to be:
- Clinical trials and researches about COVID-19 and diabetes.
- Published articles, pre-print researches, and reports of currently undergoing researches.
- No age and geographical location distinction.
The primary outcome of the study was to determine COVID-19 and diabetes relation. Electronic search was done on PubMed and Springer Link databases, with the keywords of COVID-19, epidemiological, clinical characteristics, comorbidities, and diabetes (Figure 1).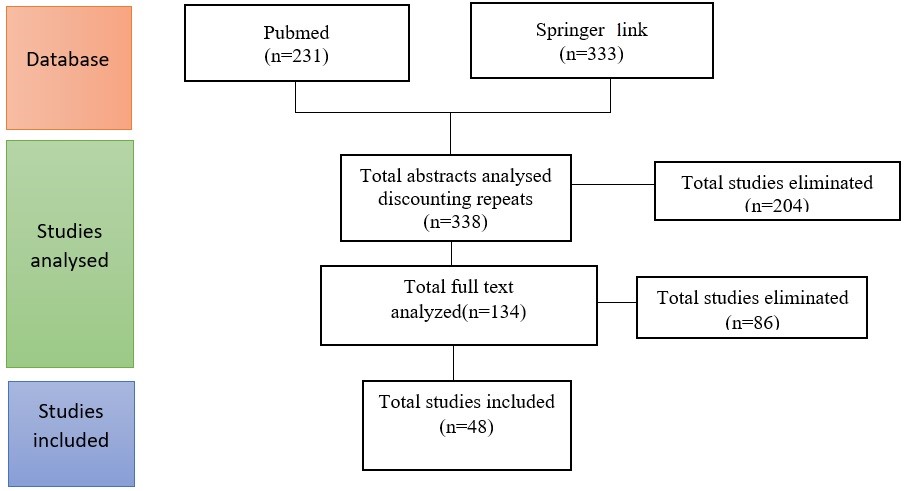
Figure 1: Flowchart detailing study selection.
The results of the databases were evaluated by the author on May 10–20, 2020. In order to minimalize the error and bias risks, this procedure was performed two times with ten days interval. To obtain the researches about the topic; the titles and abstracts were screened, duplications were detected and removed, the manuscripts of the researches which met eligibility criteria were evaluated for the detailed data, and the appropriate researches were included to the study (Figure 1).
Diabetes increases infection risk by the factors as; neutrophil dysfunction, decreased T-cell response, and poor humoral immunity [8]. Findings about diabetes and other comorbidities’ prevalence and mechanism of action in the novel COVID-19 pandemic will be important in shaping future strategies.
When the 48 included researches in this systematic review are evaluated, it is seen that; 35 have been made in China, 5 have been made in USA, 2 have been made in Italy, and there exists one study from Spain, France, Iran, Israel, South Korea and Belgium. The researches totally include 91.172 COVID-19 patients.
Diabetes prevalence in the researches differ from 3.3% to 40% [19, 44]. When the researches including only fatal cases are considered, this ratio is between 22.4% to 40% [44, 55]. WHO reports diabetes prevalence as 8.5% in adults [3]. Although the diabetic patient ratio differs in a wide range, it is under than the predicted ratio in only 11 researches, while this ratio is over 20% in 15 included researches of the review. As Gentile et al.’s findings of nearly doubled ratios of > 80 years of age diabetic patient percentages, many of the researches indicate statistically significant increased diabetes prevalence among COVID-19 patients (Table 1) [30]. Furthermore, Xiong et al. and Chow et al. have reported diabetes ratio to be higher in symptomatic COVID-19 patients, and this is an important point to focus on about diabetes and COVID-19 relation [35, 38]. Differences among the researches may be due to the included patient heterogeneity but achieved results of the review simply predicts diabetes as a potential risk factor for COVID-19 infection.
When COVID-19 infection correlation with diabetes is evaluated, it is seen that diabetes has been detected to be a risk factor in 17 of the included researches [6, 7, 13–16, 28, 31, 36, 37, 41, 45, 47, 49, 52–54]. Among them; Huang et al. have reported diabetes to be an independent risk factor and Garg et al. have shown diabetes to be a risk factor for COVID-19 severity in all ages [45, 53]. As an important point to focus on, Huang et al., Ren et al. and Deng et al. have reported diabetes to be the only risk factor for COVID-19 among other comorbidities [18, 20, 21].
In the review, diabetic COVID-19 patients’ illness severity has been compared in 18 researches (Table 2). When the mentioned researches are evaluated, it is seen that COVID-19 infection severity is statistically higher in diabetics in 8 of the researches [13, 15, 20, 27, 36, 37, 47, 53], while 4 researches did not find a significance [34, 35, 41, 46]. According to Guo et al.’s research, diabetic COVID-19 patients without other comorbidities not only had severe pneumonia risk, but also had; increased release of injury‐related enzymes, uncontrolled severe inflammatory responses, glucose metabolism dysregulation associated hypercoagulable state, and increased levels of inflammation‐related biomarkers (C‐reactive protein, IL‐6, D‐dimer, serum ferritin and coagulation index) [6]. Likewise, in their research with 7337 patients including 952 pre-existing type 2 diabetics, Zhu et al. have determined that, while diabetic patients needed more medical interventions and had multiple organ injury, well-controlled blood glucose levels (3.9 to 10.0 mmol/L) indicated lower mortality [7].
There exist several factors in determining diabetes and infection relation, as Sirtuin 1 repression and IL-17 expression [10, 58–60]. When the researches in this review are evaluated; blood glucose level regulation has been shown to be a very important determinant in many of the studies [7, 48], also Ren et al. have determined TyG index (proposed to be an insulin resistance marker) to be associated with COVID-19 severity and mortality [13].
Also, when looked from the inverse side, infections are important risk factors for acute hyperglycemia and diabetic complications [61], and there exists several reports about ketosis, hyperosmolar hyperglycemic state, and COVID-19 severity [62–64]. Likewise, among the included researches, Bode et al. have reported the hyperglycemia ratio in COVID-19 patients without a pre-diagnosis of diabetes to be nearly 20% (with increased morbidity and mortality prevalence), besides they have determined acute hypoglycemia ratios to be higher in diabetic and/or uncontrolled hyperglycemic patients [48].
There exist 9 researches about the relation of diabetes with COVID-19 mortality ratios (Table 3). Among them, in 4 of the researches diabetes has been found to increase fatality [16, 17, 23, 43], while 2 researches determined no relation [29, 51]. Besides, 6 researches, which is not included to ‘Table 3’, have stated diabetes to be related to mortality for COVID-19 infection [7, 18, 39, 42, 44, 48]. Among them, Bode et al. determined mortality ratio to be four-fold higher in diabetic and/or uncontrolled hyperglycemic patients [48].
Hypertension (HT) is known to be a risk factor for disease onset and severity of infection diseases [65, 66]. In this review HT ratios varies between 1.99% to 76% [33, 57]. However, the smallest ratio of mortality-based researches is 37.6% [55]. While 10 researches in the review have reported HT to be a risk factor for COVID-19 onset [13, 16, 17, 21, 23, 24, 27, 28, 43, 44], Wu et al. and Leung C did not find any relation [36, 39]. These findings indicate HT an important risk factor for COVID-19 infection.
Cardiovascular disease (CVD) ratios have been determined to be 1.3% to 68.7% in the included researches [35, 49]. When the researches about mortality in COVID-19 are evaluated, the smallest ratio is 11.8% [55]. CVD has been determined to be related with COVID-19 onset and severity in 8 researches [17, 21, 27, 29, 31, 35, 47, 51]. Among them; Wang et al., Deng et al. and Ruan et al. have determined CVD disease to increase COVID-19 mortality rates and Xiong et al. have determined CVD to be the only comorbidity effecting COVID-19 onset [17, 21, 29, 35].
In the evaluation of gender and age, 5 researches reported male gender [13, 16, 21, 27, 47], and 7 researches determined age as risk factors for COVID-19 infection [13, 15–17, 24, 44, 47]. While smoking rates were determined between 1.3% and 32.5%, no relation was obtained between smoking and COVID-19 disease in the included researches [38, 57]. Also, there was only one research that reported pulmonary diseases as a risk factor for COVID-19 infection [51].
When the mentioned comorbidities are evaluated as a whole, they have not only been reported to increase the onset and severity of COVID-19 infection but also have been determined to cause poorer outcomes [50, 52, 56, 57]. Also, diabetes and HT reveal as the most common comorbidities that effect the COVID-19 morbidity and mortality rates when compared with the other comorbidities. These findings show noteworthy similarities with the former studies about comorbidities, diabetes, infection, and COVID-19 [1, 8, 66–71]. However, there exists important limitations as, lack of homogeneity of patient groups and increased ratios of undiagnosed diabetes patients. By this point, specified researches about; the diabetes’ role among other comorbidities, diabetic complications, hyperglycemia ratios, insulin need and usage modalities, and new-onset diabetes in COVID-19 patients will be valuable to understand the topic in a more detailed manner.
| Author/Country | Date(day, month, year) | n | Age | DM
n (%) | HT n (%) | CVD n (%) | Smoker n (%) | RD n (%) | Ref |
| Lian J et al./China | 17.01.20-07.02.20 | 788 | 45.8 | 57 (7.23) | 126 (15.98) | 11 (1.39) | 54 (6.85) | 9 (1,14) | [12] |
| Ren H et al./China | 12.01.20-13.02.20 | 151 | 59.5 | 39 (25.8) | 60 (39.7) | 16 (10.6) | NR | 2 (1.3) | [13] |
| Yuan J et al./China | 11.01.20-04.02.20 | 94 | 40 | 5 (5.30) | 9 (9.60) | 6 (6.40) | NR | NR | [14] |
| Chen Q et al./China | 01.01.20-11.03.20 | 145 | 47.5 | 14 (9.65) | 22 (15.17) | NR | 6 (4.13) | 15 (10.34) | [15] |
| Tu W et al./China | 03.01.20-24.02.20 | 174 | 53.7 | 17 (9.77) | 37 (21.26) | NR | NR | 12 (6.89) | [16] |
| Wang D et al./China | NR-10.02.20 | 107 | 51 | 11 (10.3) | 26 (24.3) | 13 (12.1) | NR | 3 (2.8) | [17] |
| Huang Q et al./China | 17.01.20-10.02.20 | 54 | 41 | 5 (9.3) | 8 (14.8) | 8 (14.8) | NR | 2 (3.7) | [18] |
| Zhao XY et al./China | 16.01.20-10.02.20 | 91 | 46 | 3 (3.3) | NR | NR | NR | 2 (2.2) | [19] |
| Ren D et al./China | 11.01.20-12.02.20 | 150 | 54 | 12 (8) | 25 (16.7) | 8 (5.3) | 3 (2) | 4 (2.7) | [20] |
| Deng G et al./China | NR-11.02.20 | 44,672 | NR | 1102 (5.3) | 2683 (12.8) | 873 (4.2) | NR | 511 (2.4) | [21] |
| Deng G et al./China | NR-11.02.20 | 44,672 | NR | 1102 (5.3) | 2683 (12.8) | 873 (4.2) | NR | 511 (2.4) | [21] |
| Liu J et al./China | NR-16.02.20 | 24 | 57.9 | 2 (8) | 7 (29) | 3 (12.5) | 2 (8) | NR | [22]* |
| Zhao X et al./China | 07.01.20-28.02.20 | 532 | 49.5 | 59 (11.09) | 108 (20.30) | NR | NR | NR | [23]€ |
| Helms J et al./France | 03.03.20-31.03.20 | 150 | 63 | 30 (20) | NR | NR | NR | 21 (14) | [24]β |
| Pavoni V et al./Italy | 28.02.20-10.04.20 | 40 | 61 | 16 (40) | 16 (40) | 12 (30) | NR | 4 (10) | [25] |
| Zhang L et al./China | 17.02.20-19.03.20 | 20 | 71.2 | 3 (15) | 10 (50) | 4 (20) | NR | 1 (5) | [26]# |
| Shi Y et al./China | NR-17.02.20 | 487 | 46 | 29 (6.0) | 99 (20.3) | 11 (2.3) | 40 (8.2) | NR | [27] |
| Zhang J et al./China | 16.01.20-20.02.20 | 19 | 73 | 4 (21.05) | 11 (57.9) | 3 (15.8) | NR | 3 (15.8) | [28] |
| Ruan Q et al./China | NR | 150 | 57.7 | 25 (16.7) | 52 (34.7) | 13 (8.7) | NR | 3 (2) | [29]€ |
| Gentile S et al./Italy | NR | 1102 | NR | 347 (31.5) | 794 (72.1) | 302 (27.4) | NR | 202 (18.3) | [30] ⸫ |
| Lian J et al./China | 17.01.20-31.01.20 | 465 | 45 | 28 (6.02) | 82 (17.63) | 3 (0.65) | 60 (12.90) | 0 | [31] |
| Wan S et al./China | 23.01.20-08.02.20 | 135 | 47 | 12 (8.9) | 13 (9.6) | 7 (5.2) | 9 (6.7) | 1 (0.7) | [32] |
| Nikpouraghdam M et al./Iran | 19.02.20-15.04.20 | 2964 | 55.5 | 113 (3.81) | 59 (1.99) | 37(1.25) | NR | 60 (2.02) | [33] |
| Huang C et al./China | 16.12.19-02.01.20 | 41 | 49 | 8 (20) | 6 (15) | 6 (15) | 3 (7) | 1 (2) | [34] |
| Xiong F et al./China | 01.01.20-10.03.20 | 131 | 63.3 | 30 (22.9) | NR | 90 (68.7) | 39 (29.8) | 5 (3.8) | [35]α |
| Wu C et al./China | 25.12.19-26.01.20 | 201 | 51 | 22 (10.9) | 39 (19.4) | 8 (4.0) | NR | 5 (2.5) | [36] |
| Itelman E et al./Israel | 20.02.20-10.04.20 | 162 | 52 | 30 (18.5) | 49 (30.2) | 12 (7.4) | 11 (8.9) | 2 (1.2) | [37] |
| Chow N et al./USA | 12.02.20-28.03.20 | 7162 | NR | 784 (10.9) | 113 (1.57) | 647 (9.0) | 96 (1.3) | 656 (9.2) | [38]⸪€ |
| Leung C/China | 26.11.19-29.01.20 | 46 | 70.6 | 11 (26.2) | 17 (40.5) | NR | NR | 7 (16.3) | [39]⸫ |
| De Abajo et al./Spain | 01.03.20-24.03.20 | 1139 | 69.1 | 310 (27.2) | 617 (54.2) | 119 (10.5) | NR | 119 (10.5) | [40] |
| Hong KS et al./South Korea | NR-29.03.20 | 98 | 55.4 | 9 (9.2) | 30 (30.6) | 11 (11.2) | NR | 3 (3.1) | [41] |
| Richardson S et al./USA | 01.03.20-04.04.20 | 5700 | 63 | 1808 (33.8) | 3026 (56.6) | 595 (11.1) | NR | 766 (13.4) | [42]€ |
| Zhou F et al./China | 29.12.19-31.01.20 | 191 | 56 | 36 (19) | 58 (30) | 15 (8) | 11 (6) | 6 (3) | [43] |
| Li X et al./China | 14.01.20-13.02.20 | 25 | NR | 10 (40) | 16 (64) | 8 (32) | NR | 2 (8) | [44]⸫ |
| Garg S et al./USA | 01.03.20-30.03.20 | 178 | NR | 47 (28.3) | 79 (49.7) | 45 (27.8) | NR | 55 (34.6) | [45] |
| Zhang J et al./China | 16.01.20-03.02.20 | 140 | 57 | 17 (12.1) | 42 (30.0) | 7 (5.0) | 2 (1.4) | 2 (1.4) | [46] |
| Wang X et al/China | 07.02.20-12.02.20 | 1012 | 50 | 27 (2.7) | 46 (4.5) | 15 (1.5) | NR | 20 (2.0) | [47] |
| Bode B et al./USA | 01.03.20-06.04.20 | 1122 | NR | 194 (17.3) | NR | NR | NR | NR | [48] |
| Zheng Y et al./China | 16.01.20-04.02.20 | 73 | 43 | 4 (5.5) | 9 (12.3) | 1 (1.3) | 8 (10.9) | 4 (5.5) | [49] |
| Zhu L et al./China | 30.12.19-20.03.20 | 7337 | 54 | 952 (13) | 1763 (24.0) | 363 (5.0) | NR | 56 (0.8) | [7] |
| Guan W et al./China | 11.12.20-31.01.20 | 1590 | 48.9 | 130 (8.2) | 269 (16.9) | 59 (3.7) | 111 (7) | 24 (1.5) | [50] |
| Wang L et al./China | 01.01.20-06.02.20 | 339 | 69 | 54 (16.0) | 138 (40.8) | 53 (15.7) | NR | 21 (6.2) | [51]⸬ |
| Zhou X et al./China | 25.01.20-20.02.20 | 110 | 57.7 | 11(10.0) | 36 (32.7) | 10 (9.1) | NR | 3 (2.7) | [52] |
| Huang R et al./China | 22.01.20-10.02.20 | 202 | 44 | 19 (9.4) | 29 (14.4) | 5 (2.5) | 5 (2.5) | 7 (3.5) | [53] |
| Guo W et al./China | 10.02.20-29.02.20 | 174 | 59 | 37 (21.26) | 43 (24.7) | 32 (18.4) | NR | 14 (9.7) | [6] |
| Orioli L et al./Belgium | 29.02.20-26.04.20 | 11018 | NR | NR (21.2) | NR (38.5) | NR (32.9) | NR | NR (14.5) | [54] |
| Du Y et al./China | 09.01.20-15.02.20 | 85 | 65.8 | 19 (22.4) | 32 (37.6) | 10 (11.8) | NR | 2 (2.4) | [55]⸫ |
| Li T et al./China | 01.01.20-31.01.20 | 182 | 65.8 | 51 (28.0) | 29 (15.9) | 19 (10.4) | NR | 15 (8.24) | [56]⸬ |
| Palaiodimos L et al./USA | 09.03.20-12.04.20 | 200 | 64 | 79 (39.5) | 152 (76.0) | 33 (16.5) | 65 (32.5) | 28 (14.0) | [57] |
Table 1: Prevalence of diabetes, hypertension, cardiovascular disease, smoking status, and respiratory diseases in COVID-19. *: Patients with severe or fatal infection and acute exacerbations have been included, #: only tracheal intubated patients included, ⸪: hospitalized and non-hospitalized patients included, ⸫: only fatal cases have been reported, ⸬: only old patients have been included, €: evaluated according to reported number and ratios, β: only COVID-19 patients are included, α: done with hemodialysis patients, CVD: cardiovascular disease, HT: hypertension, NR: not reported, RD: respiratory disease, Ref: references, USA: United States of America.
| Author | n | DM n (%) | Mild-moderate cases n (%) | Severe cases n (%) | p value between mild-moderate and severe cases | Ref |
| Ren H et al. | 151 | 39 (25.8) | 16/89 (18.0) | 23/62 (37.1) | 0.008 | [13] |
| Yuan J et al. | 94 | 5 (5.30) | 3/75 (4) | 2/11 (18.18) | NR | [14] |
| Chen Q et al. | 145 | 14 (9.65) | 7/102 (6.9) | 7/43 (16.3) | 0.08 | [15] |
| Huang Q et al. | 54 | 5 (9.3) | 3/51 (5.9) | 2/3 (66.7) | NR | [18] |
| Zhao XY et al. | 91 | 3 (3.3) | 2/61 (3.27) | 1/30 (3.3) | NR | [19] |
| Ren D et al. | 150 | 12 (8) | 3/101 (3) | 9/49 (18.4) | 0.003 | [20]¥ |
| Shi Y et al. | 487 | 29 (6.0) | 22/438 (5.0) | 7/49 (14.3) | 0.009 | [27] |
| Wan S et al. | 135 | 12 (8.9) | 3/95 (3.1) | 9/40 (22.5) | NR | [32] |
| Huang C et al. | 41 | 8 (20) | 7/28 (25) | 1/13 (8) | 0.16 | [34] |
| Xiong F et al. | 131 | 30 (22.9) | 25/101 (24.8) | 5/30 (16.7) | 0.35 | [35]α |
| Wu C et al. | 201 | 22 (10.9) | 6/117 (5.1) | 16/84 (19.0) | 0.002 | [36] |
| Itelman E et al. | 162 | 30 (18.5) | 22/136 (16.17) | 8/26 (30.8) | 0.04 | [37]€ |
| Chow N et al. | 7162 | 784 (10.9) | 251/1037 (24)* | 148/457 (32)* | NR | [38] |
| Hong KS et al. | 98 | 9 (9.2) | 6/85 (7.1) | 3/13 (23.1) | 0.096 | [41] |
| Zhang J et al. | 140 | 17 (12.1) | 9/82 (11.0) | 8/58 (13.8) | 0.615 | [46] |
| Wang X et al. | 1012 | 27 (2.7) | 20/912 (2.2) | 7/100 (7.0) | 0.01 | [47] |
| Zheng Y et al. | 73 | 4 (5.5 ) | 1/43 (2.3) | 3/30 (10) | NR | [49] |
| Huang R et al. | 202 | 19 (9.4) | 11/179 (6.1) | 8/23 (34.8) | < 0.001 | [53] |
Table 2: Prevalence of diabetes in non-severe and severe COVID-19 patients. *: only hospitalized patients included, ¥: sepsis and non-sepsis patients are evaluated, α: done with hemodialysis patients, €: evaluated according to reported number and ratios, DM: diabetes mellitus, NR: not reported, Ref: references.
| Author | n | DM n (%) | Survivor n (%) | Non-survivor n (%) | p value | Ref |
| Tu W et al. | 174 | 17 (9.77) | 11/149 (7.4) | 6/25 (24.0) | 0.010 | [16] |
| Wang D et al. | 107 | 11 (10.3) | 6/88 (6.8) | 5/19 (26.3) | 0.024 | [17] |
| Deng G et al. | 44,672 | 1102 (5.3) | 80/406 (19.7) | 1022/20576 (5.0) | < 0.001 | [21]* |
| Zhao X et al. | 532 | 59 (11.09) | 50/503 (9.9) | 9/29 (31.0) | < 0.001 | [23]€ |
| Zhang J et al. | 19 | 4 (21.05) | 1/11 (9.09) | 3/8 (37.5) | NR | [28] |
| Ruan Q et al. | 150 | 25 (16.7) | 25 (16.7) | 12/68 (17.6) | 0.88 | [29] |
| Nikpouraghdam M et al. | 2964 | 113 (3.81) | 102/2725 (3.74) | 11/239 (4.6) | NR | [33]€ |
| Zhou F et al. | 191 | 36 (19) | 19/137 (14) | 17/54 (31) | 0.0051 | [43] |
| Wang L et al. | 339 | 54 (16.0) | 43/274 (15.8) | 11/65 (17.2) | 0.116 | [51]⸬ |
Table 3: Prevalence of diabetes in survivor and non-survivor COVID-19 patients. *: the fatality rate is detected after missing data is evaluated, ⸬: only old patients have been included, €: evaluated according to reported number and ratios, DM: diabetes mellitus, Ref: references.

