Congenital diaphragmatic hernia (CDH) is a common malformation, characterized by a defect in the diaphragm originating from incomplete fusion of pleuro-peritoneal folds (Bochdalek type) and septum transversum (Morgagni type) through which the abdominal viscera migrate into the thoracic cavity during fetal life [1]. The incidence of CDH ranges from 1 in 2500–5000 births in recent population-based studies [1]. Abnormal development of the pulmonary vasculature leads to persistent pulmonary hypertension of the newborn (PPHN) and pulmonary vasculature hyper-reactivity. Episodes of hypoxia and hypercapnia can further increase the pulmonary hypertension and lead to persistent fetal circulation [2]. With advances in the management of CDH, the overall survival has improved and has been reported to be 70–90% in non-ECMO infants and up to 50% in infants who undergo ECMO [3].
Extracorporeal membrane oxygenation (ECMO) is considered as the last life-saving option for infants ≥ 34 weeks’ gestation or with weight > 2 kg with CDH and no associated major lethal anomalies after conventional medical management has failed [4]. However, the management of CDH with ECMO remains controversial [5]. Mortality of ECMO usage is the highest in those with CDH compared to other conditions requiring ECMO, which may be reflective of the severity of illness seen in CDH [6]. Furthermore, CDH is the most common respiratory indication for ECMO [7]. Up to one-third of infants with CDH are treated with ECMO, but guidelines remain vague [8]. Multiple surveys of pediatric surgeons nationally and internationally have shown significant variations in the practice of using ECMO for CDH [9].
Reports of short and long-term outcomes, including various morbidities, are still sparse in the literature. The efficacy of ECMO in reducing mortality has not been convincing either in randomized trials [4]. Coupled with this is the fact that there are no universally accepted guidelines or consensus statements regarding ECMO indication and the optimal timing of the surgical repair for neonates managed with ECMO.
The aim of the present study is to present the experience of a high-volume specialist center with the use of ECMO in CDH patients focusing on the timing of surgery, survival outcomes, and early and long-term complications related to ECMO use and surgical repair.
A list of all neonates referred to our tertiary referral hospital with the diagnosis of CDH and treated with ECMO between 1st January 2000 and 31st December 2021 was obtained from the medical coding and audit registration section in our institution and their records were retrospectively analyzed. We used our digital hospital patient management system which records all documents, letters, communication media, and investigation findings, to collect the relevant data of individual patients. It is worth mentioning that a majority of these babies were referred from other centers specifically for consideration of ECMO, and once accepted their whole care including surgery was provided at this center until their discharge.
Patients affected by Morgagni hernia or diaphragmatic eventration were excluded from the study, as well as patients with incomplete data or who underwent the primary surgical repair in another hospital. We intentionally avoided any risk stratification of the patients or comparison with the non-ECMO group to focus only on the outcomes of CDH infants treated for initial stabilization.
In our center, there are no Trust-approved fixed protocols or criteria for the initiation of ECMO in neonates with CDH, and each case is judged individually by our specialist ECMO team in conjunction with our neonatal intensivists and pediatric cardiothoracic surgeons. The reason behind such practice is that each such neonate is complicated in its respective presentation with varied risk profiles. Thus, applying a generalized fixed set of numbers during assessment might not always be the most appropriate approach to the management of the baby. However, certain international consensus guidelines are taken into consideration when evaluating a neonate for initiation of ECMO, such as oxygenation index (OI) > 40 for 4 h, OI > 20 for 24 h with no improvement despite maximal medical therapy, refractory severe hypoxia or respiratory failure related to pulmonary hypertension and/or right ventricular dysfunction. Pediatric surgeons do not take part in these early processes and decision-making but do so when ECMO decannulation is being considered and the neonate is eventually prepared to undergo surgical repair of the diaphragmatic defect. ECMO decannulation is considered at an appropriate stage based on the status of the baby, marked by improvement in physiological parameters and clinical judgment of the treating team at the specific time. Similar to ECMO initiation, criteria for decannulation are also individualized rather than adopting a set fixed protocol. Following the successful discontinuation of ECMO, we would then aim to perform the diaphragmatic repair at the earliest opportunity when the infant is on minimal ventilatory and circulatory support. However, deviations of this general rule may apply when certain neonates are stable enough not to require ECMO pre-operatively but then need post-operative ECMO support or when infants are too unstable to be weaned off ECMO and so the repair is attempted while on ECMO.
We identified and recorded immediate (defined as any event during the initial hospital stay until discharge) ECMO-related complications and surgical adverse effects while admitted to the neonatal unit and later in a general surgical or medical ward in our center. The long-term complications/morbidities were noted from their last follow-up visits as outpatients in the various specialty clinics in our center. It is worth specifying here that no fixed time limit was chosen as the end-point but rather detailed information of all individual patients was incorporated in this study from the recorded notes including when they were last reviewed in our center. The early (within 1 year of hospital discharge) and long-term (beyond 1 year) outcomes that were considered are: mortality, hernia recurrence, pulmonary, gastrointestinal morbidities, and neurological and musculoskeletal complications.
Out of 392 patients with CDH referred and treated in our tertiary pediatric surgical center, 58 (14.8%) required ECMO within the study period.
Patients’ demographics are reported in the table (Table 1). There were discrepancies and incompleteness in the antenatal records of the older patients and thus we avoided including details of antenatal parameters in the study. Cardio-respiratory and surgical strategies adopted are presented in the tables below (Tables 2 and 3). While neonatal critical care strategies evolved and advanced over the years, ECMO has been initiated for the sickest of babies in recent times; however, we haven’t detected any specific pattern changes.
| Variables | ECMO cohort (n = 58) |
| Gender (n = 58) |
| female (n; %) | 25 (43.1%) |
| male (n; %) | 33 (56.9%) |
| Prenatal diagnosis | 31 (53.4%) |
| Mode of delivery: |
| vaginal delivery (n; %) | 36 (62.1%) |
| caesarean section (n; %) | 22 (37.9%) |
| Gestational age at birth (weeks; median, IQR) | 38 (37 – 42) |
| Birth weight (grams; median, IQR) | 2,920 (2,760 – 3,230) |
| APGAR score at 1 min (median, IQR) | 3 (2 – 5) |
| APGAR score at 6 min (median, IQR) | 5 (3 – 6.5) |
| Side of the defect: |
| right (n; %) | 18 (31.0%) |
| left (n; %) | 39 (67.2%) |
| bilateral (n; %) | 1 (1.7%) |
| Liver herniation (n; %) | 8 (13.7%) |
| Comorbidities: |
| Cardiac anomalies (n; %) | 0 No structural cardiac defects, only evidence of PHTN, bi-directional PDA flow |
| Chromosomal anomalies (n; %) | 0 |
Table 1: Patients’ demographics.
| Variables | ECMO cohort (n = 58) |
| Pre-ductal blood gas analysis |
| PaO2 (kPa; median, IQR) | 74 (26.5 – 82.5) |
| Post-ductal blood gas analysis |
| PaO2 (kPa; median, IQR) | 70 (2 – 80) |
| PaCO2 (kPa; median, IQR) | 6.72 (5.14 – 7.78) |
| pH (kPa; median, IQR) | 7.28 (7.18 – 7.42) |
| Oxygenation index (OI) (n; median, IQR) | 49.5 (38.3 – 67.4) |
Table 2: Cardio-respiratory parameters before ECMO cannulation.
| Variables | ECMO cohort (n = 58) |
| High frequency oscillation (n; %) | 58 (100.0%) |
| Nitric Oxide (n = 58) (n; %) | 58 (100.0%) |
| Ionotropes (n = 58) (n; %) | 58 (100.0%) |
| Mode of ECMO (n = 58) |
| veno-venous (n; %) | 3 (5.1%) |
| veno-arterial (n; %) | 55 (94.8%) |
| Age at initiating ECMO (n = 58) (hours; median, IQR) | 35 (22 – 48) |
| ECMO duration (n = 58) (hours; median, IQR) | 185 (129.5 – 252) |
| Age at surgery (days; median, IQR) (n = 47) | 17 (13.75 – 22) |
| Type of repair (n = 47) |
| Primary (n; %) | 26 (55.3%) |
| Prosthesis (n; %) | 21 (44.7%) |
| Type of surgery (n = 47) |
| Open (n; %) | 41 (87.2%) |
| Thoracoscopic (n; %) | 6 (12.7%) |
Table 3: Cardio-respiratory and surgical strategies adopted.
As shown in the figure (Figure 1), 47/58 (81.0%) neonates underwent the surgical repair of the diaphragmatic defect, while the remaining 11 died before the repair could be undertaken. Among these 11 neonates, 9 patients were never stable enough to be weaned off ECMO and 2 patients deteriorated rapidly soon after decannulation and eventually died before surgery. The surgical repair of the diaphragmatic defect was performed within a median of 144 h (IQR: 80 – 270) after weaning off ECMO in 42/47 (89.3%) patients, before ECMO in 4 (8.5%) infants and while on ECMO in one single (2.1%) patient. Of the 42 babies who underwent surgical repair after weaning off ECMO, 35 (83.3%) infants survived in the post-operative period. Of the 4 neonates who required ECMO only post-operatively, having been initially stable pre-operatively, 2 (50.0%) died after a mean of 27.7 days following surgery for refractory pulmonary hypertension. The only neonate undergoing the repair while on ECMO, since the neonate was not tolerating the trial-off ECMO, died on the first post-operative day from severe coagulopathy and multi-organ failure. The figures show a detailed graphical representation of the mortality figures and trends (Figures 2 and 3). The median length of hospital stay (LOS) in our institution was 33 days (IQR 16 – 47) for the 37 infants who survived surgical repair.
 Figure 1: Overall study results summary: mortality and survival numbers.
Figure 1: Overall study results summary: mortality and survival numbers.
 Figure 2: Number of deaths stratified by time of occurrence in relation to specific events.
Figure 2: Number of deaths stratified by time of occurrence in relation to specific events.
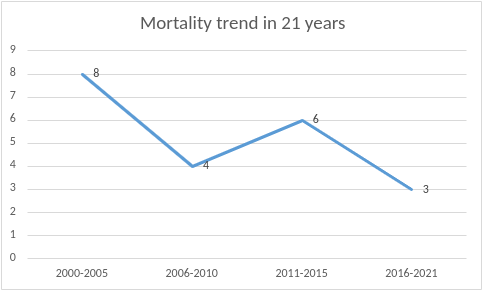 Figure 3: Mortality trends in 21 years in every 5-year block period.
Figure 3: Mortality trends in 21 years in every 5-year block period.
ECMO-related complications and early and long-term post-operative complications are listed in the table (Table 4). Pulmonary issues were the most prevalent complications in the immediate post-operative period, affecting 29 (61.7%) neonates. This was followed by gastrointestinal complications, which occurred in 4 infants (8.5%). All cases were managed successfully eventually.
| Complications | ECMO cohort (n = 58) (%) |
| ECMO-related complications |
| neurological (n, %) | 15 (25.8 %) |
| seizure (n, %) | 9 (15.5%) |
| stroke (n, %) | 3 (5.1%) |
| intracranial bleeding (n, %) | 3 (5.1%) |
| acute renal failure, anuria (n, %) | 8 (13.7%) |
| extra-cranial bleeding (n, %) | 15 (25.8%) |
| disseminated intravascular coagulation/coagulopathy (n, %) | 6 (10.3%) |
| cardio-pulmonary failure (n, %) | 7 (12.06%) |
| sepsis (n, %) | 5 (8.6%) |
| vascular (n, %) artery could not be reconstructed after decannulation | 2 (3.4%) |
| Early postoperative complications (n = 47) | |
| gastrointestinal (n, %) | 4 (8.5%) |
| necrotising enterocolitis | 1 (2.1%) |
| increased intra-abdominal pressure requiring a laparotomy | 2 (4.2%) |
| Poor feeding requiring gastrostomy | 1 (2.1%) |
| pulmonary (n, %) | 29 (61.7%) |
| pneumothorax | 14 (29.7%) |
| haemothorax | 1 (2.1%) |
| pleural effusion | 3 (6.3%) |
| chest drain insertion for complications | 2 (4.2%) |
| prolonged intubation/failed extubation | 1 (2.1%) |
| oxygen dependency | 3 (6.3%) |
| persistent pulmonary hypertension | 5 (10.6%) |
| organ failure (renal, cardio-pulmonary) | 1 (2.1%) |
| Length of hospital stay in our institution (days; median, IQR) | 33 (16 – 37) |
| Survival to discharge (n = 58) (n, %) | 37 (63.7%) |
| 1-year survival (n = 58) (n, %) | 36 (62.1%) |
| 1-year complications (n = 37) |
| hernia recurrence (n, %) | 2 (5.4%) |
| gastrointestinal (n, %) | 7 (18.9%) |
| gastroesophageal reflux | 3 (8.1%) |
| poor oral intake | 1 (2.7%) |
| poor weight gain | 2 (5.4%) |
| small bowel obstruction | 1 (2.7%) |
| neurological (n, %) | 3 (8.1%) |
| delayed motor skills | 2 (5.4%) |
| developmental delay | 1 (2.7%) |
| musculoskeletal (n, %) | 3 (8.1%) |
| pectus excavatum | 2 (5.4%) |
| thoracic scoliosis | 1 (2.7%) |
| pulmonary (n, %) | 3 (8.1%) |
| recurrent chest infection | 2 (5.4%) |
| pulmonary hypertension | 1 (2.7%) |
| Further surgeries at 1-year follow-up (n = 37) |
| re-do surgery (n; %) | 2 (5.4%) |
| Nissen fundoplication (n; %) | 2 (5.4%) |
| adhesiolysis (n; %) | 1 (2.7%) |
| Ladd’s procedure (n; %) | 2 (5.4%) |
| Last follow-up postoperative complications (n = 36): | |
| hernia recurrence (n, %) | 3 (8.3%) |
| gastrointestinal (n, %) | 8 (22.2%) |
| gastroesophageal reflux | 5 (13.8%) |
| poor oral intake/poor weight gain | 3 (8.3%) |
| neurological (n, %) | 4 (11.1%) |
| delayed motor skills | 2 (5.5%) |
| developmental delay | 1 (2.7%) |
| hydrocephalus | 1 (2.7%) |
| musculoskeletal (n, %) | 4 (11.1%) |
| chest asymmetry | 4 (11.1%) |
| pulmonary (n, %) | 4 (11.1%) |
| recurrent respiratory infections | 3 (8.3%) |
| home oxygen requirement | 1 (2.7%) |
Table 4: Early and long-term postoperative outcomes
Our in-hospital survival of CDH infants treated with ECMO was 37/58 (63.7%). The causes of death in the 10 patients who died after surgery were: severe pulmonary hypertension (n = 4; 40%), disseminated intravascular coagulation (n = 4; 40%), internal bleeding (n = 1; 10%), renal failure and anuria (n = 1; 10%).
The 1-year survival of the 37 CDH-ECMO patients who were discharged home or to their local hospital after surgery was 97.2%, one infant died at 5 months of age due to persistent and severe pulmonary hypertension.
At 1-year review, hernia recurrence was noted in 2 (5.4%) infants who needed a re-do repair within 12 months after the initial primary surgical repair. Gastrointestinal complications were the second most prevalent where feeding difficulties secondary to gastroesophageal reflux were noted in 3 (8.1%) children, of whom 2 (5.4%) required a Nissen fundoplication. Neurological complications were noted in 3 children (8.1%), followed by pulmonary complications (n = 3; 8.1%) and musculoskeletal (n = 3; 8.1%).
The total duration of follow-up from the initial discharge from our hospital was a median of 7.2 years (IQR: 11 years – 6.5 years). Few are being followed up locally and we did not have access to those notes; therefore, we considered their last visit to our center as their last follow-up for this study. There were 19 (52.9%) infants who developed long-term complications; recurrence of hernia, gastroesophageal reflux, delayed motor skills, chest asymmetry, and recurrent chest infections. 4 patients have been discharged from further review during this time.
No mortality was observed beyond 1 year of the CDH repair. This implies that the overall mortality in this series of 21 years is 36.2% (n = 21/58) and survival after surgical repair is 76.6% (n = 36/47).
This series represents a study period of 21 years involving 58 CDH neonates managed with ECMO having an overall survival of 62.0% (n = 36/58). 89.3% (n = 42/47) of the infants who underwent repair had it after discontinuation of ECMO. Pulmonary/respiratory complications (61.7%) were the most common during the initial hospital admission, while gastrointestinal morbidities (22.2%) were the most significant in the long-term. These are important data during parental counseling and while predicting prognosis for CDH babies if they require ECMO.
ECMO is a form of circulatory support used in patients with cardiac failure, respiratory failure, or both, in whom conventional therapies have been exhausted [10]. The venous blood removed is decarboxylated, oxygenated, warmed, and infused back into the venous (veno-venous (VV) ECMO) or arterial (veno-arterial (VA) ECMO) circulation. While the role of mechanical support during a respiratory failure is to allow the lungs to rest and recover; in cardiac failure the aim is to preserve end-organ perfusion while allowing myocardial rest and healing [10].
In 1975, Bartlet et al. reported the first successful use of ECMO in a newborn with cardio-respiratory failure [11]. The first use of ECMO for CDH was described by German in 1977. This report described repair of CDH on ECMO resulting in the survival of 1 out of 4 infants [12]. In 1990 ECMO was initially started in 83 centers; those numbers increased to 492 centers in 2020 [11].
The Extracorporeal Life Support Organization (ELSO) registry mentions there are about 250–300 CDH infants per year who develop severe respiratory failure requiring ECMO [7] and approximately 30% of babies with CDH are managed with ECMO therapy [7]. In our center, 14.8% CDH neonates received ECMO over 21 years, which probably reflects stricter entry criteria for ECMO utilization in our institution. It is difficult for us to make any recommendation based on these findings on whether ECMO use should be liberalized and if it has any significant true overall survival benefit since we haven’t compared it with the non-ECMO CDH cohort in this study. Moreover, this work did not analyze the risk or mortality profile of the CDH neonates treated in this institution to determine whether ECMO initiation at the specific time was always the best justifiable option for the particular neonate.
There are certain fixed contraindications when ECMO cannot be considered: weight (< 2 kg), gestational age (< 32 weeks), presence of cardiac anomalies, lethal chromosomal anomalies, or occurrence of grade III/IV intraventricular hemorrhage would preclude ECMO use [7–10, 13]. Our cohort lacked all of these criteria.
Recently in 2021, a group from University of Michigan [14] devised a Severe Pulmonary Hypoplasia and Evaluation for Resuscitation Efforts (SPHERE) protocol to help guide ECMO utilization in the most severe CDH neonates (liver up, lung-head ratio < 1, MRI O/E TFLV (total fetal lung volume) < 25%). ECMO was initiated only if the infant could obtain a pH > 7.0, pCO2 ≤100, preductal SpO2 > 80%, and PCO2 ≥40 on gentle ventilation settings for the first 2 h of life. Of the 23 patients with severe CDH included in their initial single institutional retrospective study, 57.0% fit the criteria for ECMO, with the remainder receiving comfort care only. Patient survival was 46.0% with long-term gastrointestinal, pulmonary, and neurodevelopmental sequelae seen in all.
Our overall survival rate was 62.0% (n = 36/58) in CDH babies treated with ECMO and having the hernia repaired after decannulation in the majority. As mentioned earlier, we have not utilized any such pre-defined criteria while recruiting CDH neonates for ECMO but rather relied on individual assessments of the particular patient. Nevertheless, we do feel criteria like this are worth researching further, to select the most severely affected group who will benefit the most from the timely initiation of ECMO.
While it is a rescue management strategy in the sickest CDH neonates, ECMO is not without risk. Risks include severe inflammatory response syndrome (SIRS), cannula thrombosis, machinery malfunctions, and neurological issues including seizure, intracranial hemorrhage, and infarcts from transfusion reactions and anticoagulant use [9]. Longer and repeated use of ECMO, need for inotropes, lower birth weight, prematurity, and lower Apgar scores are often responsible for higher mortality within cohorts [9]. In our surviving cohort, almost similar findings are noted, since all the surviving 37 babies developed early ECMO-related complications and 4 of them continued to have neurological manifestations in the long-term follow-up.
A USA-based nationwide database survey published in 2021 showed a mortality of neonates with CDH not requiring ECMO of 14% (153/1056) and a mortality for those requiring ECMO of 54% (72/133) [9]. Similar mortality figures have been frequently quoted in most other large series where the death rate is significantly higher for neonates requiring ECMO, with survival often ranging from 40–60% in this group [13, 14]. In our series, the overall mortality of the CDH-ECMO group was 37.9% (22/58, 11 died before repair, 10 died after repair, and 1 death 5 months after hospital discharge) which is lower than most reported figures. The mortality trend decreased over the years when the individual period of 5 years is compared as shown in the figure (Figure 3). This might be a reflection of the advancements that have taken place over the decades in neonatal critical care modalities.
It is worth mentioning that despite many uncertainties that still prevail surrounding ECMO use in CDH infants, there has never been a randomized controlled trial (RCT) conducted to determine whether the use of ECMO in CDH is beneficial [13,14]. Moreover, there is no single test or prognostication that predicts the reversibility of pulmonary hypertension, and thus criteria for ECMO referral will be variable [13, 14].
In our series, 9 (15.5%) neonates were never stable enough to come off ECMO while 2 patients deteriorated after decannulation, and all these 11 neonates (18.9%) eventually died before surgical repair could be undertaken. However, 42 infants (72.4%) did benefit from ECMO since their condition improved sufficiently to undergo decannulation and finally undergo surgical repair.
Once ECMO is initiated, the next big critical step is deciding what is the ideal timing of repair in the ECMO setting since contradictory studies and findings do exist. It has been demonstrated that physiologic deterioration occurs following CDH repair; thus, choosing the right time for repair is of paramount importance in these neonates [15]. Therefore, arguments are in place for early repair on ECMO, late repair on ECMO, or waiting till the infant is decannulated from ECMO [15]. Addressing the issue of when to operate is pivotal to understanding surgeon- or ECMO team-modifiable factors that can alter the outcome for these infants [16]. The decision to repair during an ECMO run (on-ECMO) vs. after the completion of an ECMO run (post-ECMO) is typically a discrete choice by the treating physician, institution, or ECMO team. The one exception to this is repair before ECMO (pre-ECMO), in which situation the need for ECMO is typically an unexpected event most likely secondary to postoperative disease progression [16].
Proponents of on-ECMO repair believe that repair of the diaphragmatic defect may lead to additional lung recruitment, increased thoracic space, and minimization of the risk of an unexpected second run due to peri-operative stress [16]. However, the anticoagulation required for ECMO and subsequent risk of bleeding is the primary disadvantage of this strategy and could even require premature decannulation. The timing of repair on-ECMO (early vs. late) is another complex issue with reportedly good results that vary between institutions [16]. However, those that oppose this strategy argue that repair does not necessarily help with the management of pulmonary hypertension and that post-ECMO repair is easier without the risk of anticoagulation. Unfortunately, early prediction of severity and ECMO course, although recently studied, remains challenging [16]. Without robust data to guide this decision, any anecdotally driven choice is subject to potential individual and institutional bias [16].
In the review of the CDH study group (CDHSG) database, Glenn et al. [17] identified 248 children who were repaired within 72 h of cannulation and compared them to 922 who remained unrepaired. The early repair group had improved survival to ECMO decannulation (87.1% vs. 78.4%, p-value = 0.002). The early repair group, however, did have a significantly longer median time on ECMO than the unrepaired group (240.6 vs. 196.8 h, p-value = 0.001). On the contrary, the ELSO CDH Interest Group and the CDHSG have both reported a survival benefit of late repair until after decannulation [16]. They identified 3,045 infants between 2000–2016 who were treated with ECMO and who underwent CDH repair. Of these 1,817 (59.7%) underwent repair on-ECMO while 1,228 (40.3%) underwent repair post-ECMO. The observed mortality rates for the on-ECMO and post-ECMO repair groups were 54.4% and 22.7%, respectively. The incidence of severe neurological injury was 12.6% for the on-ECMO repair group and 8.7% for the post-ECMO repair group. The odds of mortality were significantly higher in the on-ECMO repair group when compared to the post-ECMO repair group (OR: 3.41, 95% CI: 2.843-4.094, p-value < 0.0001). Robertson et al. [18] in a single institution study, suggested that delaying repair until after decannulation was associated with both improved survival and a shorter period on ECMO. Thus, our preference for repairing after ECMO decannulation is justified.
Of note, there might be a theoretical advantage for the sickest neonates who cannot be weaned off ECMO and surgical repair with decompression of the chest might be the only hope of conferring a chance of survival [19, 20]. We had one such neonate, but the baby ultimately did not survive following the repair on ECMO. Another point of debate is whether there can be a potential need for re-cannulation after repair as a result of excessive hemodynamic instability following surgery. Those repaired on ECMO thus will have an added benefit.
Currently, no RCTs exist to address the timing of repair and no doubt that it is hugely difficult due to the significant variability in disease severity, which can potentially lead to selection bias [15, 16]. The decision on best timing of repair will continue to be probably based on individual cases and institutional preferences and practice.
Very limited data exists in the published literature about early outcomes after CDH repair following ECMO decannulation. In our series, we feel operating in a much safer and stable condition after ECMO decannulation rendered our cohort to suffer only predominantly respiratory complications (61.3%) in the immediate post-operative period which were all successfully conservatively managed. The finding certainly adds arguments in favor of late repair after ECMO. Only 3 (7.3%) of our patients developed abdominal complications (increased abdominal pressure, necrotizing enterocolitis) and needed a laparotomy in the immediate post-operative period. All recovered well with no long-term consequences.
Now that CDH babies are living longer, a new cohort of survivors is providing a new insight. Patients with CDH have increased rates of neurodevelopmental disability, respiratory complications, gastrointestinal disorders, and musculoskeletal deformities [21]. Among surgical complications, hernia recurrence occurs in approximately 3–16% of all patients undergoing repair, with higher rates in certain subgroups such as minimally-invasive repair [21–23].
Despite these known risks, there is considerable variation in post-operative management between institutions, and no universal protocol for follow-up exists [21]. The need for ongoing care and lack of standardized surveillance likely contributes to frequent readmission to the hospital for this population of patients [21].
Our cohort had 1 (3.2%) case of intestinal obstruction requiring adhesiolysis and 2 patients (5.8%) had Ladd’s procedure done for malrotation within the 1st year of CDH repair. In a recent study [21], looking into 1-year outcomes after CDH repair, there were 163 (32%) patients who were readmitted within 30-days and nearly all patients were readmitted within a year (97%, n = 495). The vast majority of readmissions (96%) were unplanned and associated with complications such as gastroesophageal reflux/apparent life-threatening events (20%), CDH recurrence requiring repair (18%), admission for gastrostomy tube placement and/or fundoplication (17%). It is worth noting that since any neonatal laparotomy is associated with a 20–30% risk of bowel obstruction in later life, avoiding it through minimally invasive techniques can be explored further and there is scope to support this whenever safe and tolerated by the neonate.
In our series, recurrence was noted in 5.4% of patients (n = 2) within 1 year and another 8.1% (n = 3) in the longer term taking our overall recurrence to 13.5%, and among them, 1 recurrence occurred in the thoracoscopic group (n = 1/6, 16.7%).
In another recent 22-year review [24], 18/170 (11%) children experienced a failure of the first repair performed and among them (26%), then experienced a second failure. 2 of these 5 (40%) then failed a third time, and one child had five recurrences. The same study showed repair of recurrence has a significantly higher failure rate (32%) than the primary repair (11%). Whilst use of prosthetic patch and a thoracoscopic approach increases the probability of failure of the first repair, they have no effect on the probability of failure of repair of a recurrence [24]. Thus, our reported figures are comparable to most other recent large series.
Respiratory morbidities following CDH repair include chronic lung disease, rebound pulmonary hypertension, obstructive pulmonary disease, and infection. Treatment with ECMO and patch repair was associated with more significant pulmonary morbidity with decreased inspiratory muscle strength [21–23]. However, limited long-term data exists in the CDH-ECMO cohort since most studies highlight index admission and the events surrounding it. Nevertheless, the persistence of pulmonary hypertension and long-term oxygen dependency has been very low in our series.
Nutritional problems include gastroesophageal reflux, aversion to oral feeds, gastrostomy tube feeding, and failure to thrive in CDH survivors. In our series, 22.2% (n = 8/36) of the survivors suffered various gastrointestinal issues. In one study looking into readmissions [21], there were 36 (7%) patients with intestinal obstructions who underwent laparotomy with Ladd’s procedure during readmission. However, the ECMO group was not separately studied in this series, thus direct comparison with our results is not distinctly possible.
10% of our patients at 1 year and 13.3% in the longer term had neurodevelopmental issues. Various study findings suggest that neurodevelopmental impairment is a postnatal consequence of therapy and hypoxia from lung abnormality and is not congenital [15, 21]. Survivors of neonatal ECMO are even at higher risk due to cerebral bleeding or infarcts secondary to prolonged anticoagulation use [15]. The problems range from physical disability to neurocognitive and functional delays. Incorporation of neurological assessment in the regular review of CDH survivors with ECMO is thus of paramount importance.
Musculoskeletal issues comprised 10% of complications at 1 year and 13.3% in the long-term in our series. Several studies have described an increase in chest wall deformities following CDH repair [22, 25]. In addition, there is evidence that the more severe diaphragmatic defects are associated with a significantly greater risk of pectus deformities [25]. Survivors of CDH can thus benefit from early screening and appropriate referral to facilitate various options for orthopedic management.
![]() 2, Thiruchelvam T3, Muthialu N4, Cross K
2, Thiruchelvam T3, Muthialu N4, Cross K![]() 5, De Coppi P
5, De Coppi P![]() 5, Curry JI5, Loukogeorgakis S
5, Curry JI5, Loukogeorgakis S![]() 5, Mullaserry D5, Blackburn S
5, Mullaserry D5, Blackburn S![]() 5 and Giuliani S
5 and Giuliani S![]() *5
*5


