Neuroendocrine tumors (NETs) with oncocytic features are rare. To date, few studies have described the metastatic characteristics of these tumors, most of which were limited by the low number of cases. One article characterized intermediate to high grade (G2 and G3) oncocytic NETs of the pancreas as having an ominous outcome; however, metastatic features of low-grade oncocytic NETs have, to our knowledge, been hitherto unexplored. Our study characterized the clinicopathological and metastatic features of 32 low to intermediate-grade oncocytic NETs of various organs. The most frequent metastatic site was the liver (63%). The metastatic rate of our cases was compared with Riihimaki’s study (metastatic rate of G1/G2 NETs was reported), as well as Heetfeld’s study and Lithgow’s study (metastatic rate of G3 NETs was reported). The overall metastatic rate of our cases is 84.4%, which is significantly higher than that of the G1/2 NETs from Riihimaki’s study (84.4% vs. 25%, p < 0.0001), while similar to that of the G3 NETs from Heetfeld’s study (84.4% vs. 86.5%, p > 0.05) and Lithgow’s study (84.4% vs. 80.8%, p > 0.05). These data indicate that oncocytic features could potentially be characterized as high-risk features in addition to high Ki-67 index, mitotic count, and necrosis. Thus, imaging studies such as liver magnetic resonance imaging (MRI) may be warranted to detect liver metastases in NETs with distinctive oncocytic cytologic features even in the absence of other high-risk features.
neuroendocrine, oncocytic, metastatic, low to intermediate grade
NETs: neuroendocrine tumors; WHO: World Health Organization; GI: gastrointestinal; TC: typical carcinoids; AC: atypical carcinoids; IHC: immunohistochemical; CHR: chromogranin; SYN: synaptophysin; NSE: neuron-specific enolase; GEP-NETs: gastroenteropancreatic NETs; CT: computed tomography; MRI: magnetic resonance imaging; SSTR: somatostatin receptor; PET: positron emission tomography; ESMO: European Society for Medical Oncology
Neuroendocrine tumors (NETs) are derived from specialized cells possessing characteristics of both nerve cells and endocrine cells [1]. They occur in various organ systems due to the wide distribution of these specialized neuroendocrine cells. The most commonly involved sites are the lungs, gastrointestinal (GI) tract, and pancreas [2]. NETs are relatively uncommon, accounting for approximately 0.5% of all malignancies [3]. However, the incidence and prevalence of NETs have been increasing, with a 6.4-fold increase in the annual age-adjusted incidence rate from 1973 to 2012 [4]. The increase might be attributed to better diagnostic techniques. NETs are a spectrum of neoplasms that behave differently according to their histology and proliferation. The 2019 World Health Organization (WHO) classifies well-differentiated NETs in the GI tract/pancreas into low-grade (G1), intermediate-grade (G2), and high-grade (G3) [5]. The well-differentiated NETs in the lung/thymus are classified into typical carcinoids (TC) and atypical carcinoids (AC). TC and AC generally correspond to G1 NETs and G2 NETs. Recent studies demonstrated that G3 NETs are more frequently discovered from metastatic sites than G1/G2 NETs (2.9-fold) [6] and that prognosis is typically poor [7]. In addition to the grading system, certain morphologic variants of NETs may also affect clinical behavior and prognosis [8]. NETs with oncocytic features are tumors characterized by prominent eccentric nucleoli and abundant eosinophilic granular cytoplasm owing to abundant mitochondria. These tumors are exceedingly rare. To date, few case series and case reports have described them in the pancreas, lung, thymus, larynx, duodenum, kidney, and urinary bladder [8–16]. The outline of the metastatic characteristics of these tumors was limited by the low number of cases. One article characterized intermediate to high-grade (G2 and G3) oncocytic NETs of the pancreas as having an ominous outcome [8]; however, metastatic features of low-grade oncocytic NETs have, to our knowledge, been hitherto unexplored. Our study characterized the clinicopathological and metastatic features of 32 low to intermediate-grade oncocytic NETs of various organs.
The study was approved by our Institutional Review Board (IRB). Cases of low to intermediate-grade oncocytic NETs were retrieved from our surgical pathology database during a twenty-one-year period (2000–2021). Biopsies, resection specimens, and consultation material from outside institutions were included. Patient age, sex, primary tumor sites, metastatic sites, nuclear features, immunohistochemical (IHC) stain results, proliferation index as per MIB-1/Ki67 IHC stain, and mitosis/necrosis were extracted from the original pathology report and medical records. Tumors were graded on the basis of the current WHO classification according to their mitotic rate, Ki67 proliferation index, and, if applicable, necrosis. The metastatic rate of our cases was compared with that of a retrospective study (Riihimaki et al.) in which 7,334 patients with low to intermediate NETs of various organs were included [17]. Results were also compared to two cohort studies (Heetfeld et al. and Lithgow et al.) in which the metastatic rate of G3 NETs was reported [7, 18]. Statistical analysis: the chi-squared test was used to analyze the data (MedCalc Software Ltd). P value < 0.05 was considered to be statistically significant.
32 low to intermediate-grade oncocytic NETs cases were identified in our surgical pathology database query. Patient age, sex, primary tumor sites, metastatic sites, nuclear feature, IHC stain results, MIB-1/Ki67 proliferation index, mitosis/necrosis, and grade/classification are summarized in the table (Table 1). The mean patient age is 60.7 years (range: 29–81; 1 patient without age information was excluded). The male-to-female ratio is 11:21. The primary site of the tumor includes small bowel/ileocecum (n = 10), lung (n = 6), thymus (n = 3), stomach (n = 2), pancreas (n = 1), kidney (n = 1), and unknown (n = 9; including metastatic cases in which the origin of the primary tumor was not identified and metastatic cases without clinical history provided). 66.7% of the lung primary cases were male patients and 80% of the small bowel primary cases were female patients. 15 cases were low-grade (G1/typical) NETs with oncocytic features and 8 cases were intermediate-grade (G2/atypical) NETs with oncocytic features. The remaining 9 cases were with the unknown primary site, precluding classification into a particular grade category.
| Pt | Age | Sex | Primary site | Met site(s) | Nuclear pleomorphism | IHC | MIB-1/Ki67 | Mitosis /necrosis | Grade /Classification |
| 1 | 29 | F | Small bowel (typical) | LN | Moderate | (+) CHR, SYN, NSE, serotonin, (-)CD56 | < 1% | No | G1 |
| 2 | 44 | M | Small bowel (typical) | *Extends to the mesentery | Moderate | (+) CHR, SYN, NSE, serotonin, (focal+)CD56 | 5% | No | G2 |
| 3 | 77 | F | Small bowel (typical) | Mesenteric implants | Moderate | (+) Serotonin | < 1% | No | G1 |
| 4 | 79 | F | Ileum (typical) | LN, mesentery, peritoneum | | | < 1% | No | G1 |
| 5 | 72 | F | Ileum (typical) | LN, omentum | Moderate | | 1% | No | G1 |
| 6 | 71 | F | Terminal ileum | Liver, omentum, mesentery, b/l tube & ovary (typical) | | (+) CHR, SYN | 5% | 2 MF/10 HPF | G2 |
| 7 | 78 | F | Ileocecum (typical) | Liver, mesentery | Moderate | | 2% | No | G1 |
| 8 | 54 | F | Ileum, cecum, appendix (typical) | LN | Mild | | < 1% | No | G1 |
| 9 | 57 | F | Ileocecum, R colon | Liver (typical), LN, omentum, mesentery, peritoneum | | (+) CHR, SYN | 8% | No | G2 |
| 10 | 53 | M | Ileocecal valve (typical) | LN | Moderate to marked | | 4% | No | G2 |
| 11 | 59 | F | Lung (typical) | LN | | (+) CHR, SYN, NSE | Up to 5% | Low MF | Typical |
| 12 | 68 | M | Lung | Liver (typical), bone | Moderate | (+) CHR, SYN | < 2% | < 2 MF/10 HPF | Typical |
| 13 | 58 | M | Lung (typical) | Liver (typical) | | (+) CHR, (-) SYN | < 1% | No | Typical |
| 14 | 53 | F | Lung | NA | | (+) CHR, SYN, NSE | NA | NA | Typical |
| 15 | 64 | M | Lung | Liver (typical) | Moderate | (Focal+) serotonin | 5% | No | Typical |
| 16 | 57 | M | Lung | Liver (typical) | | (+) CHR, SYN | 5% | No | Typical |
| 17 | 70 | M | Thymus (typical) | Bone, stomach | | (+) NSE, (focal+) SYN, CD56, (-) CHR | 5–10% | No | Typical |
| 18 | 83 | F | Thymus (atypical) | NA | | (+) CHR, SYN, CD56 | 5% | 2 MF/10 HPF | Atypical |
| 19 | 40 | M | Thymus (atypical) | LN, *extends to lung & pericardium | Moderate | (+) SYN, (-) CHR | 5% | 2 MF/ 10 HPF | Atypical |
| 20 | 55 | M | Stomach (typical) | NA | | (+) CHR, SYN | NA | No | G1 |
| 21 | 70 | F | Stomach | NA | | (+) CHR, SYN | < 3% | < 2/mm2 | G1 |
| 22 | 55 | F | Pancreas | Liver (atypical) | | (+) CHR, SYN, (-) serotonin | ~20% | 7 MF/10 HPF | G2 |
| 23 | 56 | F | Kidney (atypical) | Liver, bone, b/l ovary/pelvis | | (+) CHR, SYN, NSE | NA | 3 MF/10 HPF, foci of necrosis | G2 |
| 24 | 81 | F | NA | LN (typical) | Moderate | | 6% | 1–2 MF /10 HPF | Typical |
| 25 | 51 | F | NA | Liver (typical) | | (-) Serotonin | < 1% | 1 MF/10 HPF | Typical |
| 26 | 51 | F | NA | Liver, b/l ovary, peritoneum | | | NA | Focal necrosis | Atypical |
| 27 | 47 | M | NA | Liver (atypical) | Moderate to marked | (+) CHR, SYN, NSE | 30% | 2 MF/10 HPF | Atypical |
| 28 | 52 | F | NA | Liver (atypical) | Moderate | | 10% | 2 MF/10 HPF | Atypical |
| 29 | 66 | F | NA | Liver (atypical) | Moderate | (+) CHR, SYN, serotonin | 20% | 2 MF/10 HPF | Atypical |
| 30 | 66 | F | NA | Liver (G1/G2), pancreas, bone | | (+) CHR, SYN, (-) serotonin | 8% | 1 MF/10 HPF | G1/G2 |
| 31 | 66 | M | NA | Liver (atypical) | Moderate | (+) SYN, (-) CHR | 10% | Foci of necrosis | Atypical |
| 32 | NA | F | NA | Liver (atypical) | Mild | (+) CHR | NA | > 2 MF/10 HPF | Atypical |
Table 1: Summarization of patients’ clinical and pathological characteristics of oncocytic NETs. Pt: patient; F: female; M: male; LN: lymph node; b/l: bilateral; CHR: chromogranin; SYN: synaptophysin; NSE: neuron-specific enolase; MF: mitotic figures; HPF: high power field. * Not counted for metastasis.
Microscopically, tumor cells with oncocytic features showed abundant eosinophilic granular cytoplasm, smooth nuclear membranes, and prominent nucleoli (Figures 1a and 1b). The Ki-67 proliferative index was < 3% in G1 NETs (Figure 1c) and 3–20% in G2 NETs. 2 cases demonstrated mild nuclear pleomorphism (patients 8 and 32), 12 cases showed moderate nuclear pleomorphism, and 2 cases exhibited moderate to marked nuclear pleomorphism (patients 10 and 27). 2 cases only contained focal oncocytic features (patients 11 and 32). The tumor cells generally demonstrated immunoreactivity for chromogranin (CHR) and synaptophysin (SYN) (Figures 1d and 1e). Specifically, in 20 cases in which both CHR and SYN IHC stains were performed, 16 cases were CHR+SYN+ (80%), 3 cases were CHR-SYN+ (15%), 1 case was CHR+SYN- (5%). All 7 cases stained with neuron-specific enolase (NSE) IHC stain were NSE+.
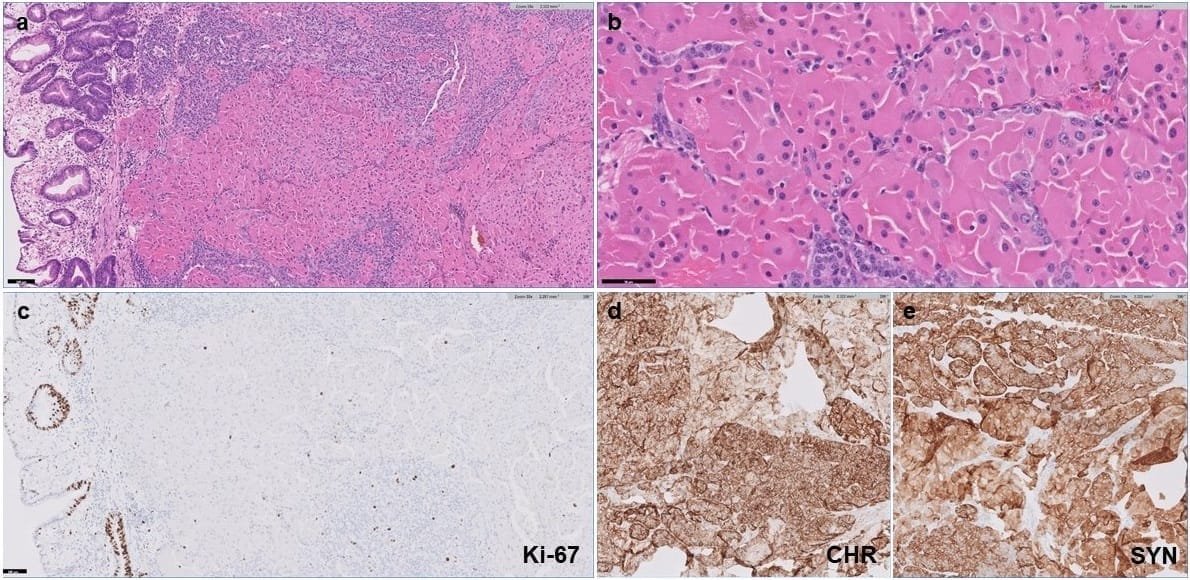
Figure 1: Low grade neuroendocrine tumor with oncocytic features. (a) H&E stain under 10X magnification showing oncocytic NET in the gastric submucosa. (b) H&E stain under 40X magnification showing tumor cells with abundant eosinophilic granular cytoplasm, smooth nuclear membranes, and prominent nucleoli. (c) The Ki-67 proliferative index is low (< 3%). (d) The tumor cells are positive for chromogranin (CHR). (e) The tumor cells are positive for synaptophysin (SYN).
As demonstrated in the table (Table 2), the overall metastatic rate is 84.4%, which is much higher than that of the G1/2 NETs from Riihimaki’s study (84.4% vs. 25%, p < 0.0001), while similar to that of the G3 NETs from Heetfeld’s study (84.4% vs. 86.5%, p > 0.05) and Lithgow’s study (84.4% vs. 80.8%, p > 0.05; not shown in table). The most frequent metastatic site is the liver (63%), followed by the lymph node (33.3%). The liver metastatic rate is lower than that of the G1/G2 NETs from Riihimaki’s study (63% vs. 82%, p = 0.01), the G3 NETs from Heetfeld’s study (63% vs. 90.6%, p = 0.01) and Lithgow’s study (63% vs. 95.2%, p < 0.01; not shown in table). The metastatic rate of low and intermediate-grade NETs did not differ significantly (80% vs. 75%, p > 0.05).
| Oncocytic G1/G2 NETs (n = 32) | G1/G2 NETs from Riihimaki’s study (n = 7334) | p value | G3 NETs from Heetfeld’s study (n = 37) | P value |
| Overall metastatic rate | 84.4% | 25%* | P < 0.0001 | 86.5% | p > 0.05 |
| Metastatic oncocytic NETs (n = 27) | Metastatic NETs from Riihimaki’s study (n = 1842) | p value | G3 NETs from Heetfeld’s study (n=32) | P value |
| Rate of metastatizing to liver | 63% | 82% | P = 0.01 | 90.6% | P = 0.01 |
Table 2: Metastatic characteristics of oncocytic G1/G2 NETs compared with Riihimaki’s study [17] and Heetfeld’s study [18]. * Calculated as number of patients with metastasis (n = 1842) divided by the total number of patients (n = 7334); though the metastatic rate is 23% in the results section of Riihimaki’s paper.
NETs with oncocytic features are rare. Only a few studies have described the metastatic characteristics of these tumors, most of which were limited by the low number of cases. Our study summarized the clinicopathological characteristics of 32 low to intermediate-grade oncocytic NETs that were diagnosed at our institution over the past 21 years.
Gastroenteropancreatic NETs (GEP-NETs)
Previous studies have shown that gastroenteropancreatic NETs (GEP-NETs) account for approximately 60–70% of all NETs [3], with the most common site being the small intestine [19]. The symptoms at the time of presentation vary by tumor location; some patients are asymptomatic and the tumor is found incidentally in imaging studies. Advanced midgut NETs (jejunum, ileum, appendix, and ascending colon) with liver metastasis can cause carcinoid syndrome, as bioactive substances such as serotonin secreted by the NETs fail to be metabolized by the liver. Common symptoms include cutaneous flushing, diarrhea, wheezing/dyspnea, and carcinoid heart disease [20]. Computed tomography (CT), magnetic resonance imaging (MRI), and somatostatin receptor (SSTR)-based integrated positron emission tomography (PET)/CT are the main diagnostic and staging imaging modalities [21]. Hepatobiliary phase liver MRI was indicated to be more sensitive in detecting liver metastases than CT or SSTR-PET/CT [22–24]. Whole-body SSTR imaging with 68Ga/64Cu-PET/CT provides high sensitivity and is recommended to be part of the tumor staging [21]. Histologically, well-differentiated GEP-NETs are classified into G1 (< 2 mitoses/2 mm2 and < 3% Ki-67 index), G2 (2–20 mitoses/2 mm2 and 3–20% Ki-67 index), and G3 (> 20 mitoses/2 mm2 and > 20% Ki-67 index). Surgical resection is the mainstay therapy even in the presence of liver metastasis [21]. No data is present to support adjuvant therapy in G1 or G2 NETs. Somatostatin analogs (such as octreotide) are used to treat symptomatic carcinoid syndrome and can also be used as anti-proliferative therapy in metastatic GEP-NETs [25, 26]. The prognosis varies depending on the site, tumor grade, and tumor stage. Patients with NETs in the small intestine have a better prognosis than in the pancreas or colon/rectum [21]. There is no consensus on postoperative surveillance. The European Society for Medical Oncology (ESMO) recommends resected G1 NETs and G2 NETs with Ki-67 < 5% be followed up with imaging every 6 months, and G2 NETs with Ki-67 > 5% be followed up every 3 months at least during the first 5 years, and then every 1–2 years for lifetime surveillance [21].
Lung NETs
Lung NETs account for 20–30% of all NETs [3]. They can be found incidentally as peripheral solitary nodules without any symptoms. More often, they grow in the central airways and cause symptoms with mass effects, such as cough, hemoptysis, chest pain, and recurrent pneumonia [27, 28]. Rarely, these tumors release bioactive amines/peptide hormones that result in paraneoplastic syndromes occur. These syndromes include carcinoid syndrome (due to serotonin, etc.), Cushing’s syndrome (due to ectopic adrenocorticotropic hormone), and acromegaly (due to ectopic growth hormone-releasing hormone or insulin-like growth factor 1). These bioactive substances can be used as serologic markers to monitor the response to treatment [27]. According to the Commonwealth NET Research Collaboration and the North American NET Guidelines for the Lung NETs, CT of the chest and bronchoscopy are the initial diagnostic tests for these tumors, and liver MRI should be used for the detection of liver metastasis [29]. Histologically, well-differentiated lung/thymus NETs is classified into TC (< 2 mitoses/2 mm2 and lacking necrosis) and AC (2–10 mitoses/2 mm2 and/or foci of necrosis). TC and AC generally correspond to low-grade (G1) NETs and intermediate-grade (G2) NETs. So far, mitotic count and necrosis are routinely reported in pathological diagnosis to grade the tumor, and mitoses can predict the prognosis of TC and AC [30]. The metastatic rate for TC is less than 5%, while that for AC is 20–30% [31]. The prognosis for TC is very good after treatment, with 10-year survival rates ranging from 82–93%. The prognosis for AC is worse, with a 10-year survival rate ranging from 35–64% [32–36]. Surgical resection is the treatment of choice for both TC and AC even in the situation of lymph node metastases [37]. Adjuvant therapy is not recommended for either TC or AC [38–40]. Surveillance should be life-long and depends on if the histology is TC or AC, and if the tumor is resected or residual/metastases [28].
NETs of the unknown primary site
NETs of unknown primary site account for approximately 10–14% of all NETs [41–43]. These cases are often discovered with liver metastases and most are from GEP origin [17]. The primary site is more likely to be in the small intestine if the patient presents with carcinoid syndrome-associated symptoms. Although other primary sites such as lung NETs can also lead to carcinoid syndrome, the incidence is much lower [44]. For well-differentiated NETs of unknown primary sites, CT/MRI, SSTR imaging with 68Ga-DOTATATE PET/CT, and IHC stains on the tissue block can help to locate the origin of the tumor [45–47]. The liver is the most common site of metastasis regardless of the origin of the primary NETs [17]. Liver metastasis in neuroendocrine neoplasms has been reported to have a worse prognosis regardless of the primary sites or histology [48].
NETs with oncocytic features
The majority of the previously reported oncocytic NETs are from pancreas and lung origin. The incidence of oncocytic features in the lung and pancreas NETs is 8% and 4.85%, respectively [9, 10]. Only one oncocytic NET case from GI origin has been reported [14]. In our 32 oncocytic NETs case series, 12 cases are from GI origin, 1 case from pancreas origin, 6 cases from lung origin, 3 cases from thymus origin, 1 case from kidney origin, and 9 cases from an unknown origin. The oncocytic NETs of unknown primary site account for 28% of all oncocytic NETs in our experience, higher than the unknown primary rate in general NETs (10–14%) discussed above. Of the two pancreatic oncocytic NET case series, one reported 7 out of 11 cases (63.6%) demonstrated lymph node/liver metastasis at the time of diagnosis, while the other one reported 5 out of 7 cases (71.4%) showed lymph node/distant metastasis at the time of surgery [8, 9]. In our cohort, there was one oncocytic pancreatic NET that had liver metastasis at the time of diagnosis. 5 out of 6 of our lung cases (83.3%) showed metastasis including during follow-up; 1 case metastasized to the lymph node, and 4 cases metastasized to the liver. The prior lung oncocytic NETs case series only reported 1 out of 15 cases as having lymph node metastasis, and distant metastasis was not mentioned [10]. The overall metastatic rate in our series is 84.4%, which is much higher than that of the G1/G2 NETs from Riihimaki’s study (25%), while similar to that of the G3 NETs from Heetfeld’s study (86.5%) and Lithgow’s study (80.8%). The most frequent metastatic site in our study is the liver (63%), lower than that of the G1/G2 NETs from Riihimaki’s study (82%) and the G3 NETs from Heetfeld’s study (90.6%) and Lithgow’s study (95.2%). We did not observe a statistically significant difference in metastatic rate between low and intermediate-grade NETs (80% vs. 75%). Whether the proportion of oncocytic components and growth patterns of oncocytic NETs can correlate with the metastatic features is unknown. A larger number of cases with these histological details is needed for further study. PET with 18F-fluorodeoxyglucose (FDG) is recommended to be used in G3 NETs due to the more avid uptake of 18FDG than low-grade NETs [45, 49]. The treatment for G3 NETs is not well established [50]. The role of surgery and adjuvant therapy remains controversial [51]. Multiple cohort studies have suggested that G3 NETs do not respond well to platinum-based chemotherapy, which is the first-line treatment for neuroendocrine carcinoma (NEC) [18, 52, 53]. Temozolomide, everolimus, and peptide receptor radionuclide therapy (PRRT) are potential treatment options for patients with G3 NETs [50]. According to the ESMO treatment guidelines for GEP-NET, chemotherapy may be effective in rapidly growing tumors or G2 NETs with a higher Ki-67 index close to G3 NETs [21]. Our study showed the metastatic rate of oncocytic G1/G2 NETs is much higher than that of the general G1/G2 NETs while similar to that of general G3 NETs, indicating oncocytic features could potentially be characterized as a high-risk feature in addition to high Ki-67 index, mitotic count, and necrosis. The application of 18FDG PET on patients with oncocytic G1/G2 NETs as well as the treatment response to chemotherapy in those patients need to be further studied.
All the authors have no direct or indirect, financial or non-financial conflicts of interest to disclose.
![]() , Liu BL
, Liu BL![]() , Mijares K
, Mijares K![]() , Han J, Paulsen JD
, Han J, Paulsen JD![]() and Sun J*
and Sun J*
