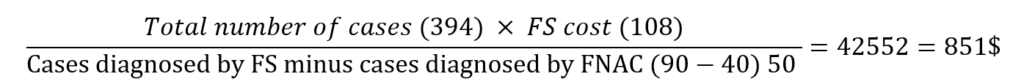Abstract
Objective: Evaluation of patients’ preferences (PP) impact on decision-making for solitary thyroid nodule management.
Study Design: A retrospective review of prospectively collected data in patients with clinical solitary nodules admitted for thyroid surgery. PP survey in various management strategies included determinants of surgery, fine-needle aspiration cytology (FNAC), frozen section (FS), and PP for total thyroidectomy (TT) or total lobectomy (TL) compared to guidelines concordance.
Results: Thyroid surgery was performed for 558 patients, 75.8% were females and 43.7% were international. FNAC was done in 79.8% and refused by 20.2% due to the misperception that it spreads cancer. The risk of malignancy was the reason for choosing surgery in 35.1%. FS was preferred by 87% of the patients for decision-making (TT vs TL) in our setting with available pathology resources and low FS cost. FS based decisions were more guideline-concordant (79%) with TT performed in 41% patients compared to 74.4% in PP based decisions alone (P < 0.001). 57.9% of the patients preferred surgeon authorization for decision-making when FS was unavailable. Papillary thyroid carcinoma (PTC) occurred in 85.3%. FS diagnosed PTC in 79% of the patients with malignant nodules in inconclusive FNACs (Bethesda I, III, IV, and V).
Conclusion: Decisions (TT vs TL) based on PP and beliefs compared to FS based decisions were less guideline-concordant (21% vs 79%) with more TT performed (74.4% vs 41%) (P < 0.001). Advancing patients’ knowledge on their disease, guidelines, and equipoise awareness is needed for better-shared decision-making.
Keywords
thyroid nodules, thyroidectomy, frozen section, fine-needle aspiration cytology, guidelines, global healthcare
Abbreviations
PP: patients’ preferences; FNAC: fine-needle aspiration cytology; FS: frozen section; TT: total thyroidectomy; TL: total lobectomy; PTC: papillary thyroid carcinoma; PPV: positive predictive value; NPV: negative predictive value; DTC: differentiated thyroid carcinoma; NIFTP: non-invasive follicular thyroid neoplasm with papillary-like nuclear features; DCE: discrete choice experiment
Introduction
Thyroid nodules are a common clinical problem and their importance rests on the increasing incidence of malignancy [1–5]. As most thyroid nodules are asymptomatic, the main challenge in their management is to guide management strategy avoiding over or under treatment [1, 6, 7]. Concurrent technological advances in thyroid nodule management led to overdiagnosis of malignancy with a subsequent unnecessary treatment specially in low-risk papillary thyroid carcinoma (PTC), where strategies are needed to help patients consider less invasive treatment options [8]. However, current thyroid guidelines regarding the extent of surgery, total thyroidectomy (TT) vs total lobectomy (TL) depends on clinical and sonographic risk stratification, cytologic category, molecular testing (when performed), clinical setting, local resources, and patients’ preferences (PP) [1, 6, 7, 9, 10]. However, few studies investigated PP [8, 11, 12]. Lee et al. [13] concluded that more research on PP is needed to incorporate patient-centered care in health services. Ahmadi et al. [11] did the first study to evaluate PP about the extent of surgery in low-risk thyroid cancer. However, the intraoperative frozen section (FS) used to be the principal tool guiding intraoperative decision-making. But in the post-Bethesda era, its role has decreased and became controversial and this controversy is ongoing regarding its additive value to fine-needle aspiration cytology (FNAC), ultrasound stratification, and molecular testing [14–16]. Recent studies recommended against FS routine use [15, 17]. However, differences in clinical practice patterns across the geographic regions exist and organizational guidelines are not in complete agreement in all issues [1].
The present study evaluates PP impact on decision-making in various management strategies including choosing surgical treatment, FNAC, intraoperative decision-making including FS utility, and preferences for TT or TL compared to guidelines concordance in patients presenting with clinical solitary thyroid nodules.
Materials and Methods
A retrospective review of a prospectively collected database was performed for patients admitted for thyroid surgery at our tertiary care academic medical center during the period from October 2014 to October 2020. Initial evaluation included demographic data as age, sex and nationality. Clinical evaluation included the risk of malignancy, rate of growth, compressive symptoms, hypo- or hyperthyroidism, radiation exposure, family history, neck physical examination, TSH measurement, and in selected cases thyroid autoantibodies and scintigraphy.
The study included consecutive patients with clinical solitary or unilateral nodular thyroid enlargement, and excluded patients with clinical palpable contralateral nodules, hyperthyroidism, hot or autonomous nodules on isotope scan, thyroid with parathyroid surgery. Counseling of patients included a survey with a face-to-face interview on PP for determinants of surgical treatment, FNAC, various intraoperative decisions including FS utility, in addition to PP for either TT or TL (Figure 1) (Table 1). Informed consent included benefits, draw-backs, expectations, and possible alternatives for management options.
Diagnostic neck ultrasonography was performed to characterize thyroid nodules and guide FNAC. The FNAC results were categorized according to the Bethesda system [18, 19]. A FS was performed at the institution. The specimens were assessed macroscopically including touch imprint cytology. A final histological examination was then performed. The results of FS were classified as (1) benign, (2) malignant, or (3) inconclusive FS and the decision was deferred to the final pathological report. TL was done for benign solitary or unilateral disease and in some cases of low-risk differentiated thyroid carcinoma (DTC) based on current thyroid guidelines [1, 7], while TT was done in case of an intraoperative finding of pathological contralateral lobe and for malignant diagnoses. When FS was inconclusive, TL or TT was done based on patients’ preoperative counseling and preferences.
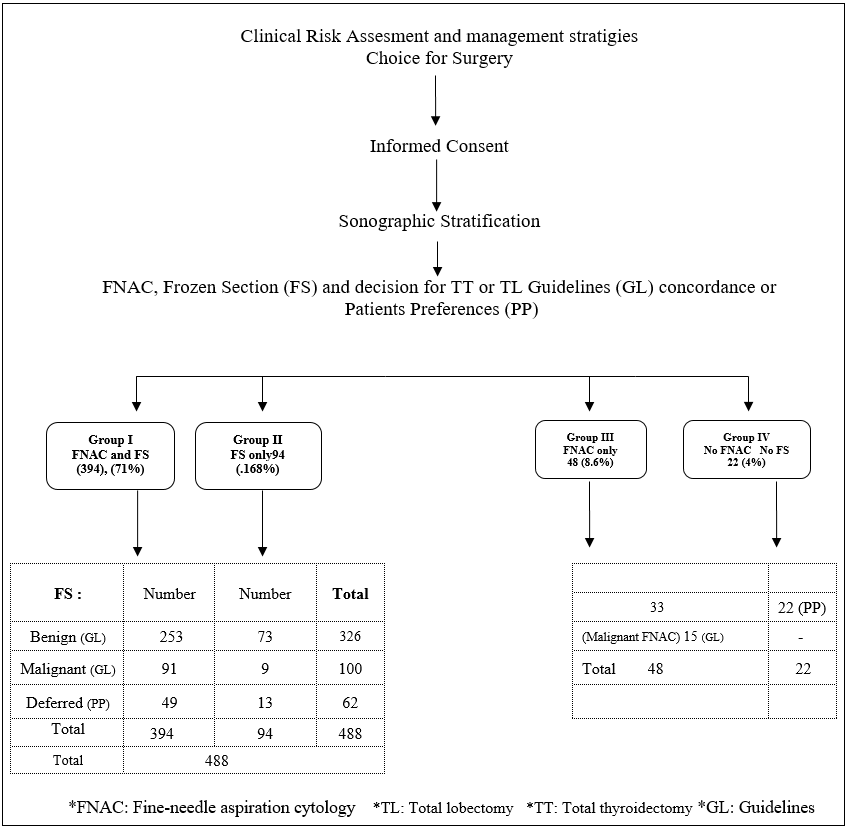
Figure 1: Clinical pathway of patients (558).
Statistical analysis was performed considering benign results as true negative for malignancy, malignant results as true positive, and deferred results were negative. Specificity, sensitivity, diagnostic accuracy, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Sensitivity was defined as true positive/true positive + false negative, specificity defined as true negative/true negative + false positive, PPV as true positive/true positive + false positive, NPV as true negative/true negative + false negative, diagnostic accuracy as true positive + true negative/total cases. The data were reported as the mean ± standard variation for continuous variables, and absolute numbers and percentages for categorical variables. Comparative analysis was performed using the χ2 test.
The cost analysis was done considering the average actual cost of FS and various surgical strategies: TL and TT with or without the cost of FS.
Results
The study included 558 patients who were admitted for thyroid surgery. Out of which 423 (75.8%) were females and 135 were males (24.2%) with a female to male ratio of 3:1. Mean age was 42.7 + 11.8 years, 45.3 + 14 years in males and 41.4 + 13.3 years in females. 314 patients were national (56.3%), while 244 were international (43.7%). Based on preoperative patient counseling and survey of PP (Table 1), determinants for surgery were risk of malignancy in 35.1%, compression symptoms in 22.9%, large nodules more than 4 cm in 19.2%, growing nodules in 18.1%, and positive family history in 5.7%. No patient had neck irradiation during childhood and no patient accepted active surveillance. FNAC was performed in 79.8% while 20.2% of the patients refused FNAC. FS was preferred by 87% of the patients to guide intraoperative decision-making, 79.2% preferred TT when FS was inconclusive, and when FS was not available 57.9% authorize their surgeon for decision-making (Table 1).
The clinical pathway of the patients is shown in the figure (Figure 1). The patients were divided into 4 groups. Group I included 394 patients in whom FNAC and FS were done. Group II included 94 patients in whom FS was done but FNAC was not done due to the patients’ misperception that FNAC would spread cancer. Group III included 48 patients in whom FNAC was done but FS was not done in 15 patients with malignant FNAC (Bethesda VI), and in 8 and 25 patients who preferred TL and TT respectively without FS. Group IV included 22 patients who preferred TL (12 patients) and TT (10 patients) without FNAC or FS (Figure 1) (Table 2).
Of all the patients (558), TL and TT were performed in 290 (51.9%) and 268 (48.1%) respectively. In 441 patients (79%), decision-making for either TL or TT was in concordance with the thyroid guidelines. While decision-making based on PP occurred in 117 (21%) patients (Table 2). TT based on PP was performed in 74.4% of the patients compared to 41% in the patients when decision-making was in concordance with the guidelines (P < 0.001) (Table 2).
| (A) Determinates for the choice of surgery |
| Risk of malignancy | 196 | (35.1%) |
| Compression symptoms | 122 | (21.9%) |
| Large nodules < 4 cm | 107 | (19.2%) |
| Growing nodules | 101 | (18.1%) |
| Positive family history | 32 | (5.7%) |
| (B) Use of FNAC |
| Yes | 445 | (79.8%) |
| No | 113 | (20.2%) |
| (C) FS utility for intraoperative extent of surgery |
| Yes | 485 | (87%) |
| No | 73 | (13%) |
| (D) FS inconclusive, patients prefer |
| Total thyroidectomy (TT) | 442 | (79.2%) |
| Diagnostic total lobectomy (TL) + completion thyroidectomy in malignancy final pathology | 116 | (20.8%) |
| (E) FS not available |
| Total thyroidectomy (TT) | 91 | (16.3%) |
| Diagnostic total lobectomy TL + completion thyroidectomy | 144 | (25.8%) |
| I authorize the surgeon to do what is suitable for me | 323 | (57.9%) |
Table 1: Preoperative patients counselling and face to face survey for preferences in surgical management of clinical unilateral nodular thyroid disease.
| TL | TT | Total | P-value |
| Preferences with guidelines (GL) concordance | < .001 |
| (GI and II) | 441 (79%) |
| Benign FS (GL) | 253 (77.6%) | 73 (22.4%) |
| Malignant FS (GL) | 7 (7%) | 93 (93%) |
| GIII |
| Malignant FNAC (GL) | – | 15 (100%) |
| Total | 260 (59%) | 181 (41%) |
| Patients’ preferences (PP) |
| Inconclusive FS (GI and II) | 10 (16.1%) | 52 (83.9%) | 117 (21%) |
| PP for TT or TL (GIII and IV) | 20 (36.4%) | 35 (66.6%) |
| Total | 290 (51.9%) | 268 (48.1%) | 558 (100%) |
Table 2: Decision-making for the extent of surgery (TL vs TT) in all patients. TL: total lobectomy; GL: guidelines; TT: total thyroidectomy; PP: patients’ preferences; F S: frozen section; FNAC: fine-needle aspiration cytology.
The outcome of the patients who refused FNAC in group II and group IV and the patients who chose TL is shown in the tables respectively (Table 3 and 4), and this was compared to the outcome of those with a shared decision with the surgeon (Table 5). The comparison showed that TL was significantly higher in patients with a shared decision with the surgeon (60%) compared to the patients who chose TL (PP) (25.6%) (P > .001).
| Decision | Final pathology | Group II (FS based) | Group IV (PP based) |
| TT 50 (43%) | Benign |
| Nodular goiter | 15 | 4 |
| Hashimoto | 7 | 3 |
| Adenoma | 6 | 1 |
| Malignant |
| Papillary | 7 | 1 |
| Follicular | 4 | 1 |
| Anaplastic | 1 | – |
| Total | 40 (42.5%) | 10 (45.5%) |
| TL 66 (57%) | Benign |
| Nodular goiter | 53 | 11 |
| Malignant |
| Papillary | 1 | 1 |
| Total | 54 (57.4%) | 12 (54.5%) |
| Total | 94 (100%) | 22 (100%) |
Table 3: Patients who refused FNAC. FNAC: fine-needle aspiration cytology; FS: frozen section; PP: patients’ preferences; TT: total thyroidectomy: TL: total lobectomy.
| Final pathology | Inconclusive frozen section (FS) (GI, GII, 62 patients) | Patients’ preferences (PP) (GIII, GIV, 55 patients) | Total |
| Benign | Nodular goiter | 9 | 16 | 25 |
| Adenoma | – | 2 | 2 |
| Malignant | Papillary | 1 | 1 | 2 |
| Follicular | – | 1 | 1 |
| Total | 10 out of (62) (38.4%) | 20 out of (55) (36.4%) | 30 |
Table 4: Patients who chose total lobectomy (TL). NG: nodular goiter; PC chose TL: papillary carcinoma; FC: follicular carcinoma; FA: follicular adenoma.
| Refused FNAC | Chose TL | Shared decision (GL) | P-value |
| GII | GIV | GI, II | GIII, GIV | GI, II, III | |
| TT |
| Benign | 28 | 8 | – | – | 73 |
| Malignant | 12 | 2 | – | – | 108 |
| Total | 40 | 10 | – | – | 181 |
| TL |
| Benign | 53 | 11 | 9 | 18 | 253 |
| Malignant | 1 | 1 | 1 | 2 | 7 |
| Total | 54 | 12 | 10 | 20 | 260 |
| 66 (out of 116) (57%) | 30 (out of 117) (25.6%) | 260 (out of 441) (60%) | P > .001 |
Table 5: Patients who refused FNAC and patients who chose TT compared to patients with shared decision. FNAC: fine-needle aspiration cytology; GL: guidelines; GI II, III, IV: group I, II, III, IV.
The final pathology in all the patients (558) was benign in 415 patients (74.4%) and malignant in 143 patients (25.6%). In benign cases, 337 were benign nodular goiter, 31 were follicular adenomas, 11 were Hurthle cell adenomas and 36 were Hashimoto. In malignant cases, 122 were PTC (85.3%) including 3 NIFTP cases, 15 were follicular carcinoma (10.5%), Hurthle cell carcinoma in one case (0.7%), anaplastic in one case (0.7%), medullary in 2 cases (1.4%), lymphoma in one 0.7%, and renal metastasis in one (0.7%).
The result of FNAC, FS, and final pathology in group I patients (n = 394) is shown in the table (Table 6). FS diagnosed PTC in 90 out of 105 patients (85.7%) with final confirmed malignancy in all categories and in 45 out of 57 patients (79 %) with inconclusive FNACs (Bethesda I, III, IV and V). But FS was not diagnostic in all follicular carcinoma, Hurthle cell carcinoma and NIFTP patients.
FNAC sensitivity, specificity, diagnostic accuracy, PPV, and NPV were 46.7%, 99.8%, 85.1%, 98.3%, and 83.1%, respectively. While FS sensitivity, specificity, diagnostic accuracy, PPV, and NPV were 84.3%, 99.7%, 95.6%,98.9%, and 94.7%, respectively.
| Frozen section (FS) | Bethesda I | Bethesda II | Bethesda III | Bethesda IV |
| Frozen | Final | Frozen | Final | Frozen | Final | Frozen | Final |
| B | M | B | M | B | M | B | M |
| Benign | 19 | 19 | 0 | 142 | 142 | 0 | 55 | 55 | 0 | 30 | 30 | 0 |
| Malignant | 2 | 0 | 2 | 6 | 0 | 6 | 7 | 0 | 7 | 9 | *1 | 8 |
| Inconclusive | 2 | 2 | 0 | 7 | 4 | 3 | 15 | 12 | 3 | 17 | 12 | 5 |
| Total | 23 | 21 | 2 | 155 | 146 | 9 | 77 | 67 | 10 | 56 | 43 | 13 |
| Frozen section (FS) | Bethesda V | Bethesda VI | Total |
| Frozen | Final | Frozen | Final | Frozen | Final |
| B | M | B | M | B | M |
| Benign | 6 | 6 | 0 | 2 | ***1 | **1 | 254 | 253 | 1 |
| Malignant | 27 | 0 | 27 | 40 | 0 | 40 | *91 | 1 | 90 |
| Inconclusive | 8 | 5 | 3 | 0 | 0 | 0 | 49 | 35 | 14 |
| Total | 41 | 9 | 32 | 42 | 1 | 41 | 394 | 289 | 105 |
Table 6: Results of FS and final pathology results in all Bethesda FNAC categories in group I. B: benign; M: malignant; FNAC: fine-needle aspiration cytology. *One false positive (FS), **One false negative (FS), ***One false positive FNAC.
The final pathology in the FS 62 deferred cases in group I and II patients was malignant in 21 cases: follicular carcinoma in 11, papillary carcinoma in 8 (including 3 NIFTP cases), medullary carcinoma in 1, and renal metastasis in 1. The final pathology was benign in 41 cases: follicular adenoma in 24, Hurthle cell adenoma in 10, and nodular goiter in 7 cases.
The cost analysis was based on the actual total cost. The cost of TL or completion thyroidectomy was (1991$), TT was (2400$), total FS cost was (108$), and TL with FS was (2064$). Two-stage thyroidectomy (initial total TL and later completion thyroidectomy if the final pathology was malignant) was (3982 $).
The cost of informative FS (group I patients) was:
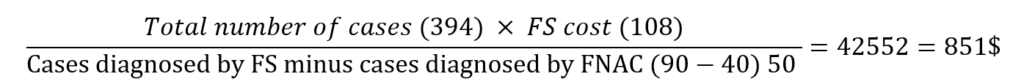
which was 42.7% of the cost of completion thyroidectomy (851$ compared to1991$).
Discussion
Current thyroid guidelines incorporated PP in decision-making [1, 6, 7, 9, 10] but they are rarely investigated, though patients’ views may differ significantly from expert opinions [11, 13]. In the present clinical setting of global healthcare, PP, availability of local resources, and cost influenced decision-making. Lee et al. [13] reported that PP in thyroid nodule management is dependent on the risk of malignancy, the prognosis of cancer, and cost. It is important that the clinicians understand what drives and determines PP and decision-making as PP can be individual driven by personal beliefs and experience [8]. A survey of PP in the present study showed that the risk of malignancy was the reason for choosing surgical treatment in 35.1% (Figure 1) (Table 1). FNAC, the initial standard of care tool in thyroid nodule management was performed in 79.8% but refused by 20.2% of the patients due to the misperception that it spreads cancer [18–20]. Discrete choice experiment (DCE) surveys showed that the disease terminology attributes of treatment, and health literacy influence decision-making [8, 11, 12, 21]. Cancer terminology can induce paradoxical decision-making for more aggressive options [8, 11, 21]. Patient-physician communication helps to ensure that PP are grounded by accurate information about preoperative evaluation, outcomes, and available treatment options. This puts the patients in the center of healthcare reducing unwarranted practice variation and sometimes cost [9, 22]. In the present study, 87% of the patients preferred FS utility to guide intraoperative decision-making (TT vs TL). The availability of pathology resources is paramount when considering FS [23, 24]. The current thyroid guidelines consider FS in the Bethesda V category [1, 7, 10], while the American Association of Endocrine Surgeons Guidelines recommended FS utilization in settings that may alter the operative procedure [6]. FS is most useful in PTC [1]. In the present study, PTC occurred in 85.3% of the patients, FS was beneficial in diagnosing malignancy in 65.9% of the patients in the Bethesda V category as compared to 50–75% in the Bethesda system, and in 79% of the patients with malignant nodules in patients with inconclusive FNACs (B I, III, IV, and V) (Table 6) [18–20]. The present study showed that the FS based decisions (TT vs TL) were guideline concordant in 79% of the patients and less invasive with TT performed in 41% as compared to 74.4% in the patients with decisions based on PP alone (P< 0.001) (Table 2) [1, 6, 7, 9, 10].
On the other hand, when FS was not available, 57.9% of the surveyed patients (Table 1) preferred to rely on their surgeon for decision-making which emphasizes the need for advancing patients’ knowledge on shared decision-making based on the understanding of their disease, guidelines, and equipoise awareness, so that they make decisions that align with their preferences [8, 12, 22].
Ahmadi et al. [11] were the first to examine PP pertaining to the extent of surgery using a DCE. Average patients preferred TT over TL for the fear of cancer recurrence with the chance of needing a second surgery after TL. However, in low-risk DTC, either TL or TT is a reasonable option [1, 7, 9, 25–30]. Both yielded comparable oncologic outcomes [28]. However, in this situation patients’ and surgeons’ preferences are important. If the patient or the surgeon chooses to do TT, then FS has no place, but if TL is the treatment of choice, then FS may be considered [31, 32]. TL was preferred in 7% of the patients with low-risk DTC in the present study. However, initial patient management decisions should be guided by perioperative risk stratification that could include the eighth edition American Joint Committee on Cancer staging system to optimize patient-specific (individualized) treatment decisions [33]. Accurate preoperative stratification can exclude advanced disease and tailor the correct initial surgical approach to avoid the need for completion thyroidectomy needed in 11–43% [12, 25, 27, 34, 35]. Moreover, the introduction of NIFTP as a tumor of low-malignant potential has an impact on management strategies for less aggressive approaches [36–38]. NIFTP has no specific cytology features and the required criteria for diagnosis are difficult to meet by FS [39, 40]. However, many studies advocated against FS utility especially in Bethesda III and IV [15, 17, 41, 42]. Neither FNAC nor FS can detect the vascular or capsular invasion required for the diagnosis of malignancy in follicular carcinoma [14, 23, 24, 41]. None of the follicular, Hurthle cell carcinoma, or NIFTP cases were diagnosed by FS in the present study. The reported limitations of the intraoperative FS include diagnostic accuracy, increased cost and time of surgery, specimen distortion which can lead to inaccurate diagnosis [15, 41]. Cotton et al. and Najah et al. [32, 42] reported that FS utility should be restricted to elderly patients, high anesthetic risk, poorly compliant for whom complementary surgery may be problematic and in patients travelling for long distances or who may come from countries where follow-up and availability of medications are limited. The present study showed that 43.7% of the patients came from other countries, looking for healthcare with minimal cost and delay [5]. The cost study of the various surgical strategies and FS in the present study was lower than that reported in the literature and informative FS cost was 42.7% of the cost of completion thyroidectomy, while two-stage surgery was the least cost-effective [41, 43–45]. Nicholson et al. [46] reported that compared to TT, two-stage thyroidectomy is likely to add cost, time, risk, and patients’ inconvenience. On the other hand, TT without FS has the disadvantage of overtreatment in benign final diagnosis, complications of TT, and lifelong hormone replacement, which are of particular importance in patients coming from abroad [47].
Nevertheless, there are limitations to our present study. It is a single institution and was not randomized. Randomized multicenter trials may be difficult to do due to the variability of institutional pathology techniques and national differences. Moreover, given the cost, large sample size, and extended time required to obtain outcomes, prospective clinical trials have not been performed [28]. However, in the absence of prospective randomized trials, we must rely on retrospective institutional and national data [48]. Molecular testing used to improve the preoperative risk assessment reducing the need for surgery or intraoperative FS was not feasible in the present setting due to the higher entailed cost, as no insurance covers these tests [6, 7, 15, 17]. Lee et al. [13] reported that while the patients prefer the added certainty of molecular tests, they are less willing to undergo these tests if they are associated with high costs. However, if thyroidectomy is indicated or preferred for clinical reasons, then molecular testing is unnecessary [6].
In summary, PP guided preoperative and intraoperative decision-making in the present clinical setting of global healthcare with low FS cost and available pathology resources. Preoperatively fear of malignancy was the reason for choosing surgery in 35.1%, avoiding FNAC in 20.2%, and non-acceptance of observation strategy. Intraoperatively 87% of the patients preferred FS to guide decisions (TT or TL). Decisions based on PP and beliefs alone compared to the decisions based on FS as an objective guiding tool were less guidelines concordant (21% vs 79%) with more TT performed (74.4% vs 41%) (P < 0.001) emphasizing the need for advancing patients knowledge on their disease, guidelines, and equipoise awareness for better-shared decision-making and consideration of less invasive treatment options.
However, future research correlating sonographic risk patterns, cytological findings, and molecular testing results may have an impact on the standardization of decision-making pertaining to the extent of surgery.
Funding
No funding was received for this study.
Availability of Data and Materials
The procedures in the current study followed have been assessed by the institutional review committee, and were in accordance with the Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients prior to initiation of the study. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors have no disclosure or conflicts that could jeopardize the current study.
Acknowledgment
Special thanks to Dr. Ahmad Al Zubaidi for statistical analysis and Mrs. Shyma Taha for editorial services.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
- Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute, Bethesda.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Nimri O AK, Assad M. Cancer incidence in Jordan -2015. Ministry of Health periodic newsletters.
- Paul H, Keckley PD, Underwood HR. Medical tourism update and implications. 2009.
- Patel KN, Linwah Y, Lubitz CC, et al. The American association of endocrine surgeons’ guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271(3):e21-e93.
- Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):622-39.
- Nickel B, Howard K, Brito JP, et al. Association of preferences for papillary thyroid cancer treatment with disease terminology: A discrete choice experiment. JAMA Otolaryngol Head Neck Surg. 2018;144(10):887-96.
- Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: What is new and what has changed? Cancer. 2017;123(3):372-81.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Thyroid carcinoma version 2. 2017.
- Ahmadi S, Gonzalez JM, Talbott M, et al. Patient Preferences around extent of Surgery in low-risk thyroid cancer: A discrete-choice experiment. Thyroid. 2020;30(7):1044-1052.
- Ramundo V, Cooper DS. Patients preferences is low-risk thyroid cancer: deciding on the extent of surgery using a discrete choice experiment. Clin Thyroidol. 2020;32(5):229-332.
- Lee DJ, Xu JJ, Brown DH, et al. Determining patient preferences for indeterminate thyroid nodules: observation, surgery or molecular tests. World J Surg. 2017;41(6):1513-520.
- Guevara N, Lassalle S, Benaim G, et al. Role of frozen section analysis in nodular thyroid pathology. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(2):67-70.
- Mallick R, Stevens TM, Winokur TS, et al. Is frozen-section analysis during thyroid operation useful in the era of molecular testing? J Am Coll Surg. 2019;228(4):474-79.
- Caraci P, Aversa S, Mussa A, et al. Role of fine-needle aspiration biopsy and frozen-section evaluation in the surgical management of thyroid nodules. Br J Surg. 2002;89(6):797-801.
- Grisales J, Sanabria A. Utility of routine frozen section of thyroid nodules classified as follicular neoplasm. Am J Clin Pathol. 2020;153(2):210-20.
- Cibas ES, Ali SZ, NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658-65.
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341-346.
- Bongiovanni M, Papadakis GE, Rouiller N, et al. The Bethesda system for reporting thyroid cytopathology explained for practitioners: frequently asked questions. Thyroid. 2018;28(5):556-65.
- Dixon PR, Tomlinson G, Pasternak JD, et al. The role of disease label in patient perceptions and treatment decisions in the setting of low-risk malignant neoplasms. JAMA Oncol. 2019;5(6):817-23.
- Stiggelbout AM, Weijden TV, Wit MPTD, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
- Almquist M, Muth A. Surgical management of cytologically indeterminate thyroid nodules. Gland Surg. 2019;8(Suppl 2):S105-S111.
- Paphavasit A, Thompson GB, Hay ID, et al. Follicular and Hürthle cell thyroid neoplasms. Is frozen-section evaluation worthwhile? Arch Surg. 1997;132(6):674-78.
- Raffaelli M, Tempera SE, Sessa L, et al. Total thyroidectomy versus thyroid lobectomy in the treatment of papillary carcinoma. Gland Surg. 2020;9(Suppl 1):S18-S27.
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol (Oxf). 2014;81Suppl:1-122.
- Vargas-Pintos S, Avenas MAR. Lobectomy compared to total thyroidectomy for low-risk papillary thyroid cancer: A systematic review. J Surg Res. 2019;242:244-51.
- Gartland RM, Lubitz CC. Impact of extent of surgery on tumor recurrence and survival for papillary thyroid cancer patients. Ann Surg Oncol. 2018;25(9):2520-525.
- Rajjoub SR, Yan H, Calcatera NA, et al. Thyroid lobectomy is not sufficient for T2 Papillary thyroid cancer. Surgery. 2018;163(5):1134-143.
- Memeh K, Ruhle B, Alsafran S, et al. Total thyroidectomy vs thyroid lobectomy for localized papillary thyroid cancer in children: A propensity-matched survival analysis. J Am Coll Surg. 2021;233(1):39-49.
- Haymart MR, Greenblatt DY, Elson DF, et al. The role of intraoperative frozen section if suspicious for papillary thyroid cancer. Thyroid. 2008;18(4):419-23.
- Cotton TM, Xin J, Sandyhya J, et al. Frozen section analysis in the post-Bethesda era. J Surg Res. 2016;205(2):393-97.
- Gulec SA, Ahuja S, Avram AM, et al. A joint statement from the American thyroid association, the European association of nuclear medicine, the European thyroid association, the society of nuclear medicine and molecular imaging on current diagnostic and theranostic approaches in the management of thyroid cancer. Thyroid. 2021;31(7):1009-1019.
- Al-Qurayshi Z, Nilubol N, Tufano RP, et al. Wolf in sheep’s clothing: Papillary thyroid Microcarcinoma in the US. J Am Coll Surg. 2020;230(4):484-91.
- Yu X-M, Wan Y, Sippel RS, et al. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(4)653-60.
- Mainthia R, Wachtel H, Chen Y, et al. Evaluating the projected surgical impact of reclassifying noninvasive encapsulated follicular variant of papillary thyroid cancer as noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Surgery. 2018;163(1):60-65.
- Amendoeira I, Maia T, Sobrinho-Simoes M. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): impact on the reclassification of thyroid nodules. Endocr Relat Cancer. 2018;25(4):R247-R258.
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023-29.
- Bongiovanni M, Giovanella L, Romanelli F, et al. Cytological diagnoses associated with noninvasive follicular thyroid neoplasms with papillary-like nuclear features according to the Bethesda system for reporting thyroid cytopathology: A systematic review and meta-analysis. Thyroid. 2019;29(2):222-28.
- Bychkov A, Keelawat S, Agarwal S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: A multi-institutional study in five Asian countries. Pathology. 2018;50(4):411-17.
- Cohen MA, Patel KR, Gromis J, et al. Retrospective evaluation of frozen section use for thyroid nodules with a prior fine needle aspiration diagnosis of Bethesda II-VI: The Weill Cornell Medical College experience. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):5-10.
- Najah H, Tresallet C. Role of frozen section in the surgical management of indeterminate thyroid nodules. Gland Surg. 2019;8(Suppl 2):S112-S117.
- Udelsman R, Westra WH, Donovan PI, et al. Randomized prospective evaluation of frozen-section analysis for follicular neoplasms of the thyroid. Ann Surg. 2001;233(5):716-22.
- Callcut RA, Selvaggi SM, Mack E, et al. The utility of frozen section evaluation for follicular thyroid lesions. Ann Surg Oncol. 2004;11(1):94-98.
- Zanocco K, Heller M, Elaraj D, et al. Cost effectiveness of intraoperative pathology examination during diagnostic hemithyroidectomy for unilateral follicular thyroid neoplasms. J Am Coll Surg. 2013;217(4):702-10.
- Nicholson KJ, Teng CY, McCoy KL, et al. Completion thyroidectomy: A risky undertaking? Am J Surgery. 2019;218(4):695-99.
- Bashir AY, Al-Zubidie AN, Bashir MA, et al. The optimal parathyroid hormone cut-off threshold for early and safe management of hypocalcemia after total thyroidectomy. Endocr Pract. 2021;S1530-891X(21):00058-6.
- Cobin RH, Gharib H, Bergman DA, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. American association of clinical endocrinologists. American college of endocrinology. Endocr Pract. 2001;7(3):202-20.