Metformin
For almost 60 years, metformin has been used to treat diabetes. The effects of metformin include decreased hepatic glucose production and increased peripheral glucose usage [14]. As well as reducing blood glucose levels, metformin may provide CV protection for multiple reasons, including weight loss, improved hemostatic function, reduced inflammation, reduced oxidative stress, and inhibiting critical steps in atherosclerosis [15]. Metformin shows CV safety according to FDA guidelines issued in 2008 [16]. The United Kingdom Prospective Diabetes Study 34 (UKPDS 34) provides the most conclusive evidence for metformin’s CV safety, which is a randomized controlled trial (RCT) with metformin that demonstrated clinically and statistically significant reductions in diabetes-related endpoints (relative risk reduction [RRR] 32%), diabetes-related death (RRR 42%), myocardial infarction (MI) (RRR 39%), and all-cause death (RRR 36%) [15].
Metformin has been shown to reduce the risk of major adverse cardiovascular events (MACE). In LEE, Kuang-Tso et al. study, the MACE rate for the metformin user group was significantly lower than that of the nonuser group (1072.0 vs. 1165.9 per 100,000 person-years, P < .001). During years 1 and 2 after the diagnosis of DM, the metformin group had a significantly lower incidence rate of MACE compared with the lifestyle modification group. Additionally, metformin taken for 12 years was associated with a significantly higher cumulative MACE-free rate than lifestyle changes (P < .001) [17].
The results of another randomized study showed that obese patients treated intensively with metformin were less likely to develop diabetes-related endpoints (32% decrease, p = 0.002), diabetes-related deaths (42%, p = 0.017), or all-cause deaths (36%, p = 0.011) when compared to conventionally treated obese patients. Sulfonylureas (SU) and insulin groups showed no reduction in risk [18]. A lack of weight gain and improved endothelial function were hypothesized to explain the benefit of metformin over insulin or SU. In addition to improving endothelial function, metformin reduces levels of plasminogen activator inhibitor 1 (PAI-1) [19].
A Cochrane review in 2005 included for analysis 29 trials that compared metformin with SU, placebo, diet, thiazolidinediones (TZDs), insulin, meglitinides, and glucosidase inhibitors; results showed that metformin improved diabetes-related outcomes and all-cause mortality significantly (p = 0.03). There was a significant benefit for obese patients [20]. Metformin use was associated with a lower risk of CV death among empagliflozin-treated patients in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME). Metformin and sodium-glucose cotransporter-2 (SGLT2) inhibitors may affect CV risk through related mechanisms [21]. On the other hand, several studies have shown no improvement in left ventricular ejection fraction (LVEF) after treatment with metformin [22, 23].
In conclusion, evidence from available studies suggests that metformin reduces the risk of CVD in people with diabetes; due to its safety and efficacy, it is generally recommended as a first-line treatment for T2DM.
Incretin-based therapies dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 receptor agonists
Dipeptidyl peptidase-4 (DPP-4) inhibitors, known as gliptins, are a class of oral diabetic medications that work by increasing the levels of incretin hormones, which help to regulate blood sugar levels by increasing insulin secretion and decreasing glucagon secretion. FDA-approved DPP-4 inhibitors include alogliptin, sitagliptin, saxagliptin, and linagliptin [24]. Besides antihyperglycemic effects, these drugs also have antihypertensive, anti-inflammatory, antiapoptotic, and immunomodulatory effects on the heart, kidneys, and blood vessels independent of the incretin pathway [25].
The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction (SAVOR-TIMI) 53 study, a RCT evaluated the CV safety of the DPP-4 inhibitor saxagliptin in patients with T2DM and established CVD compared with a placebo, saxagliptin did not increase the risk of major CV events but increased the risk of heart failure (HF) hospitalizations [26]. The study’s primary endpoint was a composite of CV death, non-fatal MI, or non-fatal ischemic stroke. In the survey, saxagliptin had no significant effect on the primary endpoint (hazard ratio [HR] 1.00; 95% confidence interval [CI] 0.89-1.12; p = 0.99). The saxagliptin group, however, had a higher incidence of hospitalization for HF than the placebo group (3.5% vs. 2.8%, respectively; HR 1.27; 95% CI 1.07-1.51; p = 0.007). A secondary endpoint of the study, MACE, was not significantly increased or decreased by saxagliptin compared to placebo (HR 1.02; 95% CI 0.94-1.11; p = 0.66). MACE incidence was similar in both treatment groups (saxagliptin group, 613 events vs. placebo group, 609) [25]. Other RCTs, including the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial, Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA), all showed no increase in the risk of major CV events compared to placebo [27–29]. TECOS and EXAMINE trials did not show any difference in hospitalization for HF [27, 28].
Generally, DPP-4 inhibitors appear to be safe for patients with T2DM regarding CV outcomes. However, the risk of hospitalization for HF may be increased with some DPP-4 inhibitors, such as saxagliptin.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) work by mimicking the effects of the incretin hormone. It acts by increasing insulin secretion from pancreatic beta cells and decreasing glucagon. In addition to their appetite suppressive effect, GLP-1RAs are attractive anti-diabetic agents due to their low hypoglycemic risk and weight loss properties. Types include exenatide, liraglutide, dulaglutide, albiglutide, and semaglutide [30].
GLP-1RAs have been studied for their effects on CVO, including mortality and CVD. Reducing atherosclerosis and CVD can be achieved by lowering plasma lipid levels and BP. Recent studies indicate that GLP-1RAs signaling may contribute to atheroprotection through its protective properties against endothelial dysfunction, anti-inflammatory effects on macrophages, and anti-proliferative actions on smooth muscle cells [31].
In a meta-analysis of 33 RCTs, GLP-1RAs were compared with insulin, placebo, and other oral hypoglycemic drugs. According to the study, no significant difference was found for the MACE outcome. Comparing GLP-1RAs with placebo or pioglitazone, a substantial reduction in MACE was observed. GLP-1RAs did not result in any difference in all-cause or CV mortality compared to other treatments. The following CV risk factors were reduced by GLP-1RAs therapy: body mass index (BMI), BP, and total and HDL cholesterol (compared with placebo, insulin, pioglitazone, and SU) [32].
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial found that liraglutide significantly reduced the risk of MACE compared to placebo in patients with T2DM at high risk for CV events. The study’s primary endpoints were CV death, non-fatal MI, and non-fatal stroke. It was found that liraglutide significantly reduced the risk of the primary endpoint compared to placebo (HR 0.87; 95% CI 0.78-0.97; p = 0.01 for superiority). The incidence of the primary endpoint was 13.0% in the liraglutide group and 14.9% in the placebo group. Although insignificant, the liraglutide group had fewer hospitalizations due to HF than the placebo group [33].
On the other hand, the Exenatide Study of Cardiovascular Event Lowering (EXCEL) trial found no significant reduction in the risk of MACE with exenatide extended-release compared to placebo in patients with type 2 diabetes. Exenatide extended-release did not significantly reduce the risk of the primary endpoint compared to placebo (HR 0.91; 95% CI 0.83-1.00; p = 0.06 for non-inferiority and p = 0.12 for superiority). The incidence of the primary endpoint was 11.4% in the exenatide extended-release group and 12.2% in the placebo group. Further, exenatide extended-release did not increase the risk of HF hospitalization when compared to placebo [34].
There is a more significant promise with the results of the recently published Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) trial. The trial found that once-weekly semaglutide significantly reduced the risk of MACE compared to placebo in patients with T2DM. The study found that semaglutide significantly reduced the risk of the primary endpoint compared to placebo (HR 0.74; 95% CI 0.58-0.95; p < 0.001 for non-inferiority and p = 0.02 for superiority). The study demonstrated reductions in MACE at 6.6% in the semaglutide group vs. 8.9% in the placebo group over two years, p = 0.02. A side note: In the semaglutide group, rates of new or worsening nephropathy were lower, but complications of retinopathy (vitreous hemorrhage, blindness, or conditions requiring intravitreal agents or photocoagulation) were significantly higher [35].
These studies suggest GLP-1RAs may be safe for patients with T2DM. Regarding CVO, studies have shown that certain GLP-1RAs can reduce major CV events. However, the risk of hospitalization for HF may be slightly increased with some GLP-1RAs, such as exenatide (Figure 1).
 Figure 1: The figure demonstrates the efficacy of glucagon-like peptide-1 receptor agonists (GLP-1RAs) agents on major adverse cardiovascular events (MACE) compared to placebo based on hazard ratio results.
Figure 1: The figure demonstrates the efficacy of glucagon-like peptide-1 receptor agonists (GLP-1RAs) agents on major adverse cardiovascular events (MACE) compared to placebo based on hazard ratio results.
Sodium-glucose cotransporter-2 inhibitors
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of medications that have potent anti-diabetic effects. They lower blood glucose levels by increasing urinary glucose excretion. Currently, three drugs in this class are approved for use by the FDA and the European Medicines Agency (EMA), including empagliflozin, dapagliflozin, and canagliflozin. Aside from lowering glucose levels, SGLT2 inhibitors have several CV benefits [36, 37].
Empagliflozin was evaluated in a study designed to examine its CV safety and efficacy in T2DM patients with underlying CVD. The empagliflozin group had a lower rate of the primary MACE outcome than the placebo group (HR 0.86; 95% CI 0.74-0.99; p = 0.04). There was a 38% reduction in the risk of CV death, whereas there were no significant between-group differences in the rates of MI or stroke. Compared to the control group, there was a 38% reduction in the risk of CV death but no significant differences in MI or stroke rates. It was found that death from any cause was reduced by 32%, as well as hospitalization for HF, which was reduced by 35% [38].
SGLT2 inhibitors have improved LVEF and left ventricular mass index (LVMI). The Effects of Empagliflozin on Cardiac Structure in Patients with Type 2 Diabetes (EMPA-HEART) trial has shown empagliflozin to decrease LVM (assessed by cardiac magnetic resonance) after six months in patients with T2DM and a history of MI or previous coronary revascularization. SGLT2 inhibitors may decrease supply-demand mismatch ischemia after MI and improve LV remodeling [39]. Similar results have been seen with dapagliflozin. The trial results showed that LV diastolic function for T2DM patients with stable HF had significantly improved six months after the administration of dapagliflozin. Other LV diastolic functional parameters, such as left atrial volume index (LAVI) and LVMI, also improved six months after the administration of dapagliflozin [40].
The Dapagliflozin Effect on Cardiovascular Events (DECLARE–TIMI 58) study is a randomized, double-blind, multicenter study. The study’s results suggest that dapagliflozin provides significant CV and renal benefits in patients with T2DM. Dapagliflozin reduced the risk of MACE by 17% compared to placebo (HR 0.83; 95% CI 0.73-0.95; p = 0.005). A significant reduction in HF hospitalizations was principally responsible for the reduction in MACE (HR 0.73; 95% CI 0.61-0.88; p = 0.0008). A significant decrease in the composite renal endpoint of estimated glomerular filtration rate (eGFR) by 40%, end-stage renal disease (ESRD), or kidney or CV death was also shown with dapagliflozin (HR 0.76; 95% CI 0.67-0.87; p = 0.001) [41, 42].
Similarly, the Canagliflozin Cardiovascular Assessment Study (CANVAS) demonstrated significant reductions in CV death, non-fatal MI, and hospitalization for HF. The risk of the primary outcome was significantly lower in the canagliflozin group than in the placebo group (26.9 vs. 31.5 events per 1000 patient-years; HR 0.86; 95% CI 0.75-0.97; p < 0.001 for non-inferiority and p = 0.02 for superiority). In addition, it showed a 40% reduction in the eGFR, the need for renal replacement therapy, or death from renal causes (HR 0.60; 95% CI 0.47-0.77; p < 0.001). In the canagliflozin group, however, the incidence of adverse reactions, including genital mycotic infections and diabetic ketoacidosis, was higher than in the placebo group [43]. Other benefits included weight loss (by ~ 2 lbs), lowered systolic BP (by ~3 mmHg without increasing heart rate), reduced HbA1c (by 0.5%), and small increases in both LDL and HDL cholesterol [21].
Overall, studies of SGLT2 inhibitors suggest significant CV and renal benefits in patients with T2DM who have either established CVD or multiple CV risk factors (Figure 2).
 Figure 2: The figure demonstrates the efficacy of sodium-glucose cotransporter-2 (SGLT2) inhibitors on major adverse cardiovascular events (MACE) compared to placebo based on hazard ratio results.
Figure 2: The figure demonstrates the efficacy of sodium-glucose cotransporter-2 (SGLT2) inhibitors on major adverse cardiovascular events (MACE) compared to placebo based on hazard ratio results.
Insulin secretagogues, sulfonylureas
Sulfonylureas (SU) bind to the US receptor on beta-cells, thus triggering insulin secretion from the pancreas and decreasing HbA1c. There are several types of SU used to treat T2DM, including first-generation SU (e.g., chlorpropamide, tolazamide, and tolbutamide) and second-generation SU (e.g., glipizide, glyburide, and glimepiride) [36].
A two-fold increase in hypoglycemic-related hospitalizations was associated with SU [44]. As severe hypoglycemia is linked to an increased risk of macrovascular events, including arrhythmias and CV deaths, hypoglycemia is a significant concern [45]. In patients with and without underlying CVD, SU had a higher CVD risk than metformin [46]. SUs were associated with an increased risk for CVD, non-fatal MI, and stroke compared to insulin alone or combined with metformin in the BARI 2D trial [47].
A meta-analysis of 30 RCTs compared SU to placebo or other diabetes medications. It was found that significant CV events or CV mortality did not differ between the treatment group and the placebo group. Major CV events were not significantly increased when compared to an active comparator.
Significant increases in all-cause mortality and severe hypoglycemia were found, but it is unclear whether hypoglycemia contributed to the increase in all-cause mortality [48]. Compared with DPP-4 inhibitors, SU are associated with an increased risk of stroke, but no significant difference in the risk of CV events between the two treatment groups [49, 50]. A comparison of different SU suggests that longer-acting versions (e.g., glibenclamide) and earlier generations (tolbutamide) may pose more significant CV risks [51].
There is also evidence indicating that the duration of SU use is important. In women with diabetes, a longer course of SU treatment increases their risk of CHD [52]. However, RCT has not proven this association, and all CV risk increases with diabetes duration. Overall, CVO associated with SU use can vary depending on the type of medication used and the patient population studied.
Thiazolidinediones
Thiazolidinediones (TZDs) are a class of anti-diabetic drugs that lead to increased insulin sensitivity and decreased hepatic glucose output. There are two types of TZDs: pioglitazone and rosiglitazone [35]. The CV safety of TZDs has been questioned, particularly concerning HF and MI. TZDs increase weight gain, fluid retention, and HF. The results of 40 months observational study were an increase in the incidence of HF from 5.3% in controls to 8.2% in the TZD patients [53].
The Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study included 5,238 patients and followed them for a median of 34.5 months. In the study, pioglitazone had no significant effect on the primary endpoint of MACE when compared to placebo (HR 0.90; 95% CI 0.80-1.02; p = 0.095). Nevertheless, pioglitazone significantly reduced the risk of all-cause mortality, non-fatal MI, and stroke as secondary endpoints (HR 0.84; 95% CI 0.72-0.98; p = 0.027) [54]. Additionally, in a meta-analysis of 19 trials, the pioglitazone group showed a trend to lower rates of MI (HR 0.81; 95% CI 0.64-1.00), as well as a lower composite of death, non-fatal MI, or non-fatal stroke (HR 0.82; 95% CI 0.72-0.94) [55]. In terms of hospitalization for HF, the PROactive study found that pioglitazone was associated with a higher risk of hospitalization for HF compared to placebo (HR 1.41; 95% CI 1.14-1.76, p = 0.002) [55].
On the other hand, rosiglitazone has been associated with an increased risk of CV events, particularly MI. According to the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study, which showed a neutral effect for MACE, there was no significant difference in the composite endpoint of CV death, non-fatal MI, or stroke between rosiglitazone and metformin or sulfonylurea (HR 0.99; 95% CI 0.85-1.16, p = 0.87); however study showed an excess of eight cases of MI (both fatal and non-fatal) in the rosiglitazone group, which gives rise to an HR of 1·14 with a wide CI, which is not statistically significant. Accordingly, the evidence regarding rosiglitazone’s potential risk of MI compared to controls is inconclusive [56]. In 2007, the FDA warned about the CV risks associated with rosiglitazone, and its use was restricted in 2010; as a result of a critical meta-analysis of 42 trials, an odd ratio was reported for MI of 1.43 (95% CI 1.03-1.98), and an abnormal ratio for CV death of 1.64 in rosiglitazone treated groups compared with comparator groups (placebo, metformin, Su, and insulin) [57]. HF causing admission to hospital or death occurred in 61 people in the rosiglitazone group and 29 in the active control group (HR 2·10; 1·35-3·27, risk difference per 1000 person-years 2·6, 1·1-4·1) [56].
The studies above indicate that, despite their initial popularity, they remain relatively low in use. These drugs should be avoided in patients with HF or at high risk of HF.
Insulin
Several studies have investigated the relationship between insulin and CVO. Insulin resistance is associated with several metabolic disorders, such as dyslipidemia, HTN, and obesity, that increase the risk of CVD [58].
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial investigated the effects of intensive glucose-lowering therapy using insulin on CVO in T2DM and found that insulin did not significantly reduce the risk of major CV events compared to standard treatment [59]. In the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) study, the trial found no significant difference in the incidence of CV events between patients treated with insulin glargine and those treated with standard care for six years (HR 1.02; 95% CI 0.94-1.11, p = 0.63) [60].
Furthermore, in the Diabetes Control and Complications Trial (DCCT), type 1 diabetics were followed for 6.5 years, and found that intensive insulin therapy reduced the development and progression of microvascular disease (retinopathy, neuropathy, and nephropathy) without significantly reducing CV events [61].
Clinical trials have so far disproved any CV harm caused by insulin despite reported adverse in vitro activity. Therefore, insulin appears to be neutral in terms of MACE. A summary of the effects of various classes of antihyperglycemic medications on the classical 3-point MACE (which is defined as a composite of non-fatal stroke, non-fatal MI, and CV death) and the effect on HF hospitalizations from randomized clinical trials is shown in the table (Table 1) (Figure 3).
| Class | Individual drugs | Effect on MACE | Effect on HF hospitalizations |
| Biguanides | Metformin | ↓ a | Not assessed |
| DDP-4 inhibitors | Saxagliptin | ⇔ | ↑ |
| Alogliptin | ⇔ | ⇔ |
| Sitagliptin | ⇔ | ⇔ |
| GLP-1RAs | Liraglutide | ↓ | ⇔ |
| Semaglutide | ↓ | ⇔ |
| Exenatide weekly | ⇔ | ⇔ |
| SGLT2 | Empaglifilozin | ↓ | ↓ |
| Canaglifilozin |
| Dapaglifilozin |
| Sulfonylureas | Chloropromide | ⇔ | Not assessed |
| Glebinclemide |
| Glipizide |
| TZDs | Rosiglitazone | ⇔ | ↑ |
| Pioglitazone | b | ↑ |
| Insulin | | ⇔ | Not assessed |
Table 1: Summary of cardiovascular effects of various classes of antihyperglycemic medications from randomized controlled trial. DPP-4: dipeptidyl peptidase-4; GLP-1RAs: glucagon-like peptide-1 receptor agonists, HF: heart failure; MACE: major adverse cardiovascular events; MI: myocardial infarction; SGLT2: sodium-glucose cotransporter-2; TZDs: thiazolidinediones. a: small study, low-risk populations, MI only; b: secondary outcome.
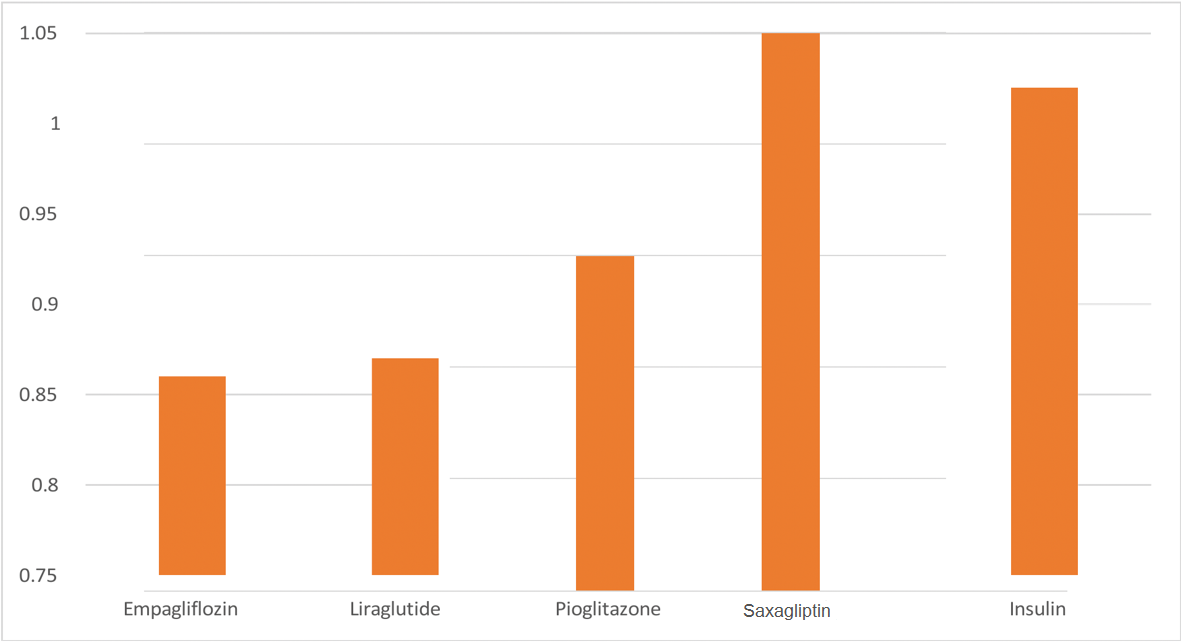
Figure 3: The figure demonstrates the efficacy differences on major adverse cardiovascular events (MACE) by a selection of anti-diabetic agents from different classes compared to placebo based on hazard ratio results.
Older adults
A significant impact on population health and economics is occurring due to the rapid growth of elderly patients with diabetes [62]. Over one-quarter of people over 65 have diabetes, and one-half of older adults have prediabetes. The number of older adults living with these conditions is expected to increase rapidly in the coming decades [63, 64]. In Saudi Arabia, 5.6% of the population is over 60 years old [65].
Microvascular and macrovascular complications of diabetes are more prevalent in older adults. These include amputations of the lower extremities, MI, end-stage renal disease (ESRD), and visual impairments [63]. There is a unique mechanism for CVD in the older population, as shown in the figure and table (Figure 4 and Table 2) [66].
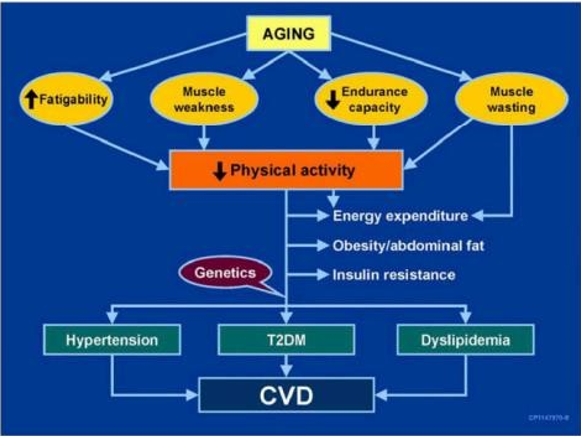 Figure 4: The figure illustrates one connection between aging, muscle, diabetes, and cardiovascular disease [66].
Figure 4: The figure illustrates one connection between aging, muscle, diabetes, and cardiovascular disease [66].
 Table 2: The table is showing the age-related changes in the cardiovascular system [66].
Table 2: The table is showing the age-related changes in the cardiovascular system [66].
- Metformin
It is the first-line agent for older adults with T2DM [67]. In one systematic review done for patients with a median age of 60 and above, in the T2DM subgroup, 18 studies reported the pooled HR was 0.83 [95% CI 0.77, 0.88] (p < 0.00001), I2 = 60%. Thus, metformin was associated with a lower CV event rate in diabetic patients than in those who did not take it [68].
- Thiazolidinediones
TZDs, if used at all, should be used very cautiously in older adults in those with or at risk for HF [67, 69, 70].
- Insulin secretagogues
SU and other insulin secretagogues are associated with hypoglycemia and should be used cautiously [67]. In one study, it was identified 339 incident cases of CVD, including 191 cases of CHD and 148 cases of stroke. A longer duration of SU use was significantly associated with a higher risk of CHD (p for trend = 0.002) [52].
- Incretin-based therapies
Oral dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 receptor agonists (GLP-1RAs):
DPP-4 inhibitors have few side effects and minimal risk of hypoglycemia and do not reduce or increase significant adverse CVO [71]. Across the trials of this drug class, there appears to be no interaction by age group [72, 73]. A challenge of interpreting the age-stratified analyses of this drug class and other CV outcomes trials is that while most of these analyses were prespecified, they needed to be powered to detect differences.
One systematic review that involved a total number of 157,478 participants with T2DM was included. Treatment with DPP-4 inhibitors did not significantly increase CVO in these patients with T2DM, indicating that those drugs might be safe to use in terms of CV events [74].
GLP-1RAs have demonstrated CV benefits in people with diabetes with established atherosclerotic cardiovascular disease (ASCVD) and those at increased ASCVD risk. New studies are investigating their benefits in other populations [71]. Systematic reviews and meta-analyses studies of GLP-1RAs trials have found that these agents reduce MACE, CV deaths, strokes, and MI equally in people over and under 65 [74, 75]. These benefits are markedly found in liraglutide and semaglutide.
- Sodium-glucose cotransporter-2 inhibitors
SGLT2 is administered orally, which may be convenient for older adults with diabetes. As well as providing CV benefits to patients with ASCVD, these agents have also proven effective for patients with HF [71]. This class of agents has also been found to be beneficial for people with HF. The stratified analyses of the trials of this drug class indicate that older adults have similar or more significant benefits than younger people [41, 76]. While understanding of the clinical benefits of this class is evolving, side effects such as volume depletion, urinary tract infection (UTI), and worsening urinary incontinence may be more common among older people. This medication is approved to be beneficial in the reduction of CV mortality, improvement in HF-related health status, and preventing MACE [77, 78].
This medication shows benefits in reducing the risk of atrial fibrillation (AF) in one nationwide cohort study assessing the risk of AF among more than 200 000 Medicare beneficiaries with T2DM; after propensity score matching, the initiation of SGLT2 is associated with an 18% decrease in the risk of AF compared with DPP-4 inhibitors and a 10% decrease in the risk of AF compared with GLP-1RAs [79].
- Insulin therapy
Around 30% of patients with HF and DM are treated with insulin. Recent post-hoc analyses of clinical trials in patients with HF with reduced and preserved LVEF found that insulin was associated with a higher risk of all-cause mortality and HF hospitalization [80, 81].
In another study comparing insulin with other drugs, 34,376 individuals aged 50 and over with DM and HF were included; 42.0% were older than 80, and 46.7% were women. As compared to insulin, SGLT2 inhibitors and GLP-1RAs significantly reduced MACE and death (SGLT2 inhibitors, HR (95% CI) 0.29 (0.23-0.36); GLP-1RAs, 0.482 (0.51-0.42), and hospitalization for HF (0.57 (0.40-0.81) and 0.67 (0.59-0.76). In patients with DM and HF, SGLT2 inhibitors and GLP-1RAs significantly reduced MACE compared with insulin, particularly any cause of death and first hospitalization for HF. These groups of medications had high safety profiles compared with other AHAs, particularly with insulin. The inadequate optimization of HF and DM cotreatment in the insulin cohort is noteworthy [82].
Compared to OHAs, insulin therapy was associated with higher mortality rates. In patients with HF, insulin therapy was detrimental, especially when HbA1c levels were low, which suggests the use of specific insulin-management strategies and blood sugar targets may be necessary [83].
Diabetes mellitus population with chronic kidney disease
Chronic kidney disease (CKD), urinary albumin excretion is persistently elevated (albuminuria), the eGFR is low, or other kidney damage symptoms are present [84, 85]. CKD due to diabetes, known as diabetic kidney disease (DKD), occurs in 20–40% of people with diabetes [84, 86–88].
In the case of T2DM and established CKD, choosing a glucose-lowering medication requires special consideration, such as limited medications available when eGFR is decreased and a desire to avoid the progression of CKD, CVD, and hypoglycemia [89, 90]. Modifications to medication dosage may need to be made accordingly if eGFR < 60 mL/min/1.73 m2 [84]. Patients with DM and CKD are at high risk for CV events and mortality [91].
- Metformin
In general, the revised FDA guidance states that 1) metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2, 2) eGFR should be monitored while taking metformin, 3) the benefits and risks of continuing treatment should be reassessed when eGFR falls to < 45 mL/min/1.73 m2, 4) metformin should not be initiated for patients with an eGFR < 45 mL/min/1.73 m2, and 5) metformin should be temporarily discontinued at the time of or before iodinated contrast imaging procedures in patients with eGFR 30–60 mL/min/1.73 m2 [84].
In one trial, 591 individuals used metformin at baseline and 3447 non-users. Among propensity-matched users, the crude incidence rate for mortality, CV mortality, CV events, and the combined endpoint was lower in metformin users than non-users. Still, ESRD was marginally higher (4.0% vs. 3.6%). Metformin use was independently associated with a reduced risk of all-cause mortality (HR 0.49; 95% CI 0.36-0.69), CV death (HR 0.49; 95% CI 0.32-0.74), the CV composite (HR 0.67; 95% CI 0.51-0.88), and the kidney disease composite (HR 0.77; 95% CI 0.61- 0.98). Associations with ESRD (HR 1.01; 95% CI 0.65-1.55) were insignificant. Results were qualitatively similar in adjusted analyses of the entire population. Two cases of lactic acidosis were observed [92].
- Thiazolidinediones
In one systematic review of the five trials (n = 233) reporting HF, one compared TZD vs. SU, and the remaining four compared TZDs vs. placebo/no additional medications. One trial used rosiglitazone as an intervention, and the other three used pioglitazone as an intervention. Meta-analysis showed that TZDs treatment did not increase the risk of HF (RR 0.64, 95% CI 0.15-2.66, I2 = 0%).
In three trials, three angina events occurred in 168 patients, all of whom were treated with pioglitazone. Upon pooling these trials, there were no statistically significant differences between pioglitazone treatment and control for the angina risk factor (RR 1.45, 95% CI 0.23-8.95; I2 = 0%). MI were reported in two trials, but no event occurred in each group, while strokes were not reported in either trial. According to one trial (RR 0.33, 95% CI 0.01-7.82) and one cohort study15 (RR 1.23, 95% CI 0.87-1.75), CV mortality may not be adversely affected by TZDs treatment. Meanwhile, five trials involving 878 participants reported all-cause mortality, with three trials comparing TZDs with active drugs (SU, DPP-4, metformin) and two trials comparing TZDs with placebos. There was no increase in all-cause mortality risk associated with TZDs (RR 0.40, 95% CI 0.08-2.01; I2= 0%) in a meta-analysis of two cohort studies (n = 3,133). Furthermore, TZDs did not increase mortality risk compared to controls (0.78, 95% CI 0.38-1.59; I2 = 85%) [93].
- Insulin secretagogues
SU monotherapy is associated with a higher risk for all-cause mortality, major hypoglycemic episodes, and CV events compared with metformin. Although the presence of CKD attenuated the mortality benefit, metformin may be a safer alternative to SU in patients with CKD [94].
- Incretin-based therapies
Several recent studies have shown CV protection from GLP-1RAs. As GLP-1RAs reduce risks of CVD events and hypoglycemia and may also slow CKD progression, they are suggested for CV risk reduction if such risk is predominant [95].
- Sodium-glucose cotransporter-2 inhibitors
Several recent studies have shown CV protection from SGLT2 inhibitors as renal protection from SGLT2 inhibitors [96]. A significant complication of CKD is HF, which can be reduced with SGLT2 inhibitors [97].
- Insulin therapy
Insulin therapy may increase CV risk and mortality among T2DM patients in several recently reported clinical outcomes trials [62].
Bariatric surgery (BS) or metabolic surgery (MS) has emerged in the last decades as a treatment for obesity and diabetes for better life quality control by dramatically reducing weight and improving glycemic control, lipid profile, and BP [98]. There is strong and consistent evidence that obesity management can delay the progression from prediabetes to type 2 diabetes [99, 100] and is highly beneficial in treating type 2 diabetes [101, 102]. In people with type 2 diabetes and overweight or obesity, modest weight loss improves glycemia and reduces the need for glucose-lowering medications [101–103], and larger weight loss substantially reduces A1C and fasting glucose and has been shown to promote sustained diabetes remission through at least 2 years. The duration of T2DM defines macrovascular and microvascular lesions and the prognosis of the BS [104–107].
In one RCT research done on Swedish obese individuals, a controlled intervention survey was used, which studied the impact of BS on T2DM following an MS. The survey associated BS with a reduction in MI. As such, 38 events among all the 345 obese patients, vs. 43 events among 262 patients in the control group, having the log-rank p = 0.017; and the adjusted HR 0.56 (95% Cl 0.34-0.93; p = 0.025) were examined [108]. The outcomes showed no effect of BS on stroke incidences. The impact of BS on reducing MI prevalence was stronger among individuals having a higher serum total cholesterol, as well as triglycerides at a baseline level, where the interaction p-value = 0.02 in both the traits. BMI (of interaction p-value = 0.12) was not associated with the surgery outcome. BS seems like a remedy to reduce incidences of MI among obese patients who have T2DM. It is therefore efficient to integrate preoperative BMI with the metabolic parameters to maximize the benefits accruing from BS [109–112]. The RCT of BS in T2DM demonstrates a significant reduction in the requirement for antihypertensive medication to lower and control BP compared to those medically treated. For instance, the STAMPEDE shows absolute variations in systolic as well as diastolic BP. At the 5-year follow-up, it was -3 ± 23 as well as -6 ± 13 mmHg at the RYGB + IMT arm, -8 ± 20 as well as -8 ± 15 in the VSG + IMT sleeve, and -4 ± 20 and -4 ± 11 in the IMT arm separately. There was no significant difference among the groups that were observed. BS lowered the death rate by CV events [113, 114]. Moreover, in the baseline study, all three types of BS reduced the chances of albuminuria when follow-up was emphasized. Additionally, the occurrence of microvascular and macrovascular outcomes reduced by performing a BS [115–118].
BS leads to enhanced CV by other mechanisms as well. Reduction in weight will lead to adipose tissue mass and systemic inflammation that will lead to improved peripheral insulin confrontation. There is a reduction of plasma leptin after an obese patient with T2DM undergoes a BS, thereby losing weight. The decline of leptin secretion lowers HTN and tachycardia which are due to the activation of the sympathetic nervous system. This leads to lower BP after BS [119, 120]. In summary, BS (or MS) improves the risk of CVD by different mechanisms, which include decreasing the CVD risk factors.
Furthermore, obesity and HF are two well-correlated disease epidemics. However, current published studies debate whether BS improves the survival rate in a patient with HF, as obesity is considered a risk factor for mortality postoperatively [121]. On the other hand, BS has been showing benefits in patients with HF, including improved symptoms, hospitalization rate reduction, and lower mortality rates [122, 123].
Moreover, health issues are reported after BS on earlier and longer terms. Due to the increased parasympathetic and reduced sympathetic activity after significant weight loss, sinus bradycardia and orthostatic intolerance were significantly found after BS, and their peak corresponded to the magnitude of BMI reduction [124, 125]. Ristow et al. demonstrated two case reports of patients considered for end-stage HF and cardiac transplantation [121]. However, due to morbid obesity, BS has been preferred, and they showed marked improvement in LV systolic function [126]. Controversy and nutritional deficiency of vitamins and minerals have been linked to cardiomyopathic processes, especially selenium deficiency, associated with life-threatening dilated cardiomyopathy. Eventually, the awareness of long-term parenteral nutritional supplies of minerals must be contemplated [127]. BS lowered the long-term risk of new-onset AF. Weight loss and BS may reduce the long-term possibility of AF [128].
The antihyperglycemic agent has multiple side effects like hypoglycemia, weight gain, etc. Severe hypoglycemia is defined as a blood glucose level < 50 mg/dL (2.8 mmol/L) and has been identified to be one of the strongest predictors of macrovascular events, adverse clinical outcomes, and mortality in people with T2DM [126]. Severe blood glucose drop indirectly activates the autonomic nervous system. It results in sympathoadrenal system stimulation, leading to the release of catecholamines, in addition to prolongation of cardiac repolarization and the QT interval, which may increase the risk of cardiac arrhythmias, including ventricular tachycardia and AF [127]. There is ample evidence that hypoglycemia is a frequent adverse complication of glucose-lowering treatment of diabetes, particularly with SU and insulin [126].
SU were associated with a 5-fold higher risk of severe hypoglycemia, mainly linked to their propensity to cause CV insult [128, 131]. These CV adverse effects are palmed to be due to interfering cardio-protective mechanisms during unselectively blocking ATP-sensitive potassium channel receptors located in the myocardial and vascular smooth muscle cells, the similar receptors SU drugs exert their action in the pancreatic β-cells to induce insulin secretion [131]. Adverse consequences on CV health were not observed with all medications in SU class; several randomized trials revealed that in comparison with glibenclamide, gliclazide was associated with a lower risk of CV-related mortality (RR 0·60, 95% CI 0·45-0·84) [132].
Other cardiovascular disease benefits/risks profile for anti-diabetic medications
On the other hand, various other anti-diabetic drugs have a significant protective action on the CV system. Much literature has been published on metformin’s CV safety profile. A meta-analysis of 40 studies proved that metformin significantly reduced CV mortality, all-cause mortality in patients with MI (aHR = 0.79) and HF (aHR = 0.84) and incidence of CV events in T2DM patients with CAD, with more superiority to SU and no significant incidence of LVEF alteration [68]. These results are consistent with previous systematic review studies that revealed no increased risk for morbidity and mortality with metformin use in patients with HF, including those with reduced LVEF or CKD [133].
Like metformin, pioglitazone may exhibit CV benefits. A large pioneer RCT involving patients with T2DM and CVD has proven significant reduction in the primary, secondary composite endpoint of all-cause mortality, non-fatal MI, and stroke in the pioglitazone group (HR 0·84; 95% CI 0·72-0·98, p = 0·027) [127, 134]. However, pioglitazone may exacerbate HF due to its ability to induce edema and fluid retention [135].
Recently, literature has already drawn attention to the paradox in novel anti-diabetic drugs, including GLP-1RAs, SGLT2 inhibitors, and DPP-4, in the context of CV system protection. A meta-analysis of 48 studies revealed that GLP-1RAs and SGLT2 inhibitors are more significantly associated with improved LVEF and left ventricular end-diastolic diameter (LVEDD), respectively. In contrast, DPP-4 inhibitors are more strongly associated with a negative impact on left ventricular end-diastolic volume (LVEDV) compared to placebos [136], which is consistent with a previous study [137].
![]() 1,6,8,9, AlSubaiee M1,6,7,8, Alshaikh Husain M
1,6,8,9, AlSubaiee M1,6,7,8, Alshaikh Husain M![]() 2, Alkhazmari G3, AlQahtani A
2, Alkhazmari G3, AlQahtani A![]() 1, Aldossary I4,6, Gado W
1, Aldossary I4,6, Gado W![]() 1, Albahrani Z1, AlMukhaylid S
1, Albahrani Z1, AlMukhaylid S![]() 5, Alarfaj A2, Almekhloof S4,6, Alarfaj M1, AlTaweel M
5, Alarfaj A2, Almekhloof S4,6, Alarfaj M1, AlTaweel M![]() *1,6 and AlMusaad A6
*1,6 and AlMusaad A6




