Chawda N1, Jain S2, Solanki B3, Tejani V4, Patel P4, Sonkar C4* and Bhattacharya AK4
1Department of Medicine, SBKS Medical Institute and Research Centre, Piparia, Gujarat, India
2Department of Medicine, Bombay Hospital and Medical Research Centre, Mumbai, India
3Department of Medicine, B.J. Medical College, Ahmedabad, Gujarat, India
4Department of Medicine, Parul Institute of Medical Sciences and Research (PIMSR), Parul Sevashram Hospital, Parul University, Limda, Waghodia, Vadodara, Gujarat, India
*Correspondence: Chetan Sonkar, Department of Medicine, Parul Institute of Medical Sciences and Research (PIMSR), Parul Sevashram Hospital, Parul University, Limda, Waghodia, Vadodara, Gujarat, India
Received on 29 October 2022; Accepted on 08 December 2022; Published on 31 December 2022
Copyright © 2022 Chawda N, et al. This is an open-access article and is distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Aim and Objective: To study cardiovascular manifestations in hypothyroidism.
Materials and Methods: An observational study was carried out in Parul Sevashram Hospital, Vadodara, Gujarat, India. 150 patients, both men, and women, diagnosed with hypothyroidism attending the Parul Sevashram Hospital were recruited from the outpatient and in-patient departments of medicine. Clinical profiles, history, complications, and all required data were collected. All patients were regularly called for follow-up based on their visits. As per the investigator’s discretion, laboratory tests, i.e., thyroid function tests, CBC, electrocardiogram (ECG), and 2D echocardiography were performed as and when required, and medication was prescribed. All physical examinations and all vitals were recorded at every visit till the end of the study.
Results: A total of 150 patients were included in this study. Male:female: 57:93; age: 28–76 years. Patients were examined in a prospective manner and results were compared with the control group to evaluate the effect of hypothyroidism, subclinical and overt, on the cardiac status by echocardiography. Variables of heart structure and function were assessed by cross-sectional and Doppler echocardiography. Interventricular septum (IVS) dimensions were significantly raised in moderate subclinical and severe overt hypothyroidism (mean 0.9 +/- 0.03 and 0.9 +/- 0.2 cm). Left ventricular posterior wall (LVPW) thickness was significantly increased only in overt hypothyroidism (mean 1.3 +/- 0.2). However, RVW and LVID showed no definite pattern of change. Pericardial effusion and diastolic dysfunction were seen in 72 cases only in overt hypothyroidism. Diastolic dysfunction with pericardial effusion was found in 74 (49.3%) cases followed by diastolic dysfunction in 49 (32.6%), systolic dysfunction in 15 (10.0 %), and increased interventricular septum spectrum thickness in 12 (8.6%) patients. The majority of the diastolic dysfunction was mild dysfunction associated with females. No cases were found to have severe diastolic dysfunction.
Discussion: On the basis of a case history, the clinical and para-clinical manifestations of hypothyroidism are reviewed. Exertion dyspnea without signs of cardiac insufficiency occurs frequently. The minute and stroke volume and heart rate are reduced. The blood pressure may rise (reversible) and hypertension may occur. The function of the left ventricle is reversibly reduced. X-ray of the thorax may reveal massive relatively asymptomatic pleural effusion and cardiomegaly. Pericardial exudate occurs frequently and is demonstrated best by echocardiography. The plasma concentrations of several different enzymes (including creatine kinase (CK), CK-MB, and LDH) may be raised in myxedema. The reason for this is perhaps compromised membrane function in the skeletal muscle cells.
Conclusion: Hypothyroidism, both subclinical and overt, is associated with cardiovascular alteration, both structural and functional. IVS and LVPW thickness are markedly affected, and there is an impairment in left ventricular function in diastole. Furthermore, hypothyroidism is more common in females, between the age group of 20–50 years. The majority of the patients have cardiovascular changes such as ECG abnormalities, pericardial effusion, diastolic dysfunction, and diastolic hypertension. We strongly suggest early detection and initiation of hormone replacement therapy can minimize the associated cardiovascular changes.
Recommendation: To study inter-and intracellular deposits, infiltrations, and fibrosis in the myocardium and these probably contribute to some of the on-specific, reversible ECG changes (low voltage, flattening/inversion of T waves, sinus bradycardia). To study hypothyroidism present can increase atheroma formation. The patients can be grouped into overt and subclinical hypothyroid.
Keywords
cardiovascular, echocardiography, hypothyroidism, impairment, manifestation, subclinical
Abbreviations
CBC: complete blood count; ECG: electrocardiogram; IVS: interventricular septum; LVPW: left ventricular posterior wall; RVW: right ventricular width; LVID: left ventricular internal diameter end diastole; LDH: lactate dehydrogenase; TSH: thyroid stimulating hormone; EDRF: endothelium-derived relaxing factor; RBS: random blood sugar; HbA1c: haemoglobin A1c; CRP: C-reactive protein
Introduction
The cardiovascular system is sensitive to the action of the thyroid hormone. However, a wide spectrum of cardiac abnormalities has long been recognized in patients with over thyroid dysfunction. Most clinical studies have shown that subclinical hypothyroidism or hyperthyroidism is associated with changes in several cardiac parameters – that patients have resting left ventricular diastolic dysfunction evidenced by delayed relaxation and impaired systolic dysfunction on the effort that results in poor exercise capacity [1]. Indeed, the at-rest left ventricular systolic function was substantially normal in most studies of subclinical hypothyroid patients compared to normal control subjects. Therefore, it would seem appropriate to initiate timely treatment for patients with mild thyroid failure to prevent cardiac involvement (Figure 1).
Thyroid dysfunction is a frequent endocrine disorder influencing about 300 million people worldwide with over half the estimated population to be unaware of their condition [2]. In accordance with a projection from various published studies on thyroid disease, same has been estimated that about 42 million people in India endure thyroid disease [3].
More than 12% of the U.S. population will develop a thyroid condition during their lifetime. An estimated 20 million Americans have some form of thyroid disease. In continuation, up to 60% of those with thyroid disease are unaware of their condition. Women are five to eight times more likely than men to have thyroid problems. One woman in eight will develop a thyroid disorder during her lifetime. Most thyroid cancers respond to treatment, although a small percentage can be very aggressive. Furthermore, the causes of thyroid problems are largely unknown. Additionally, undiagnosed thyroid disease may put patients at risk for certain serious conditions, such as cardiovascular diseases, osteoporosis, and infertility. Pregnant women with undiagnosed or inadequately treated hypothyroidism have an increased risk of miscarriage, preterm delivery, and severe developmental problems in their children. Most thyroid diseases are life-long conditions that can be managed with medical attention [4].
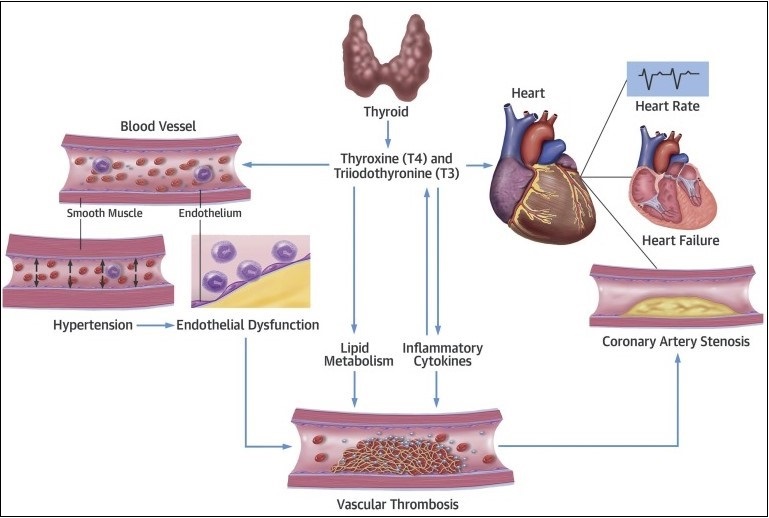 Figure 1: Interaction between thyroid hormones and cardiovascular system.
Figure 1: Interaction between thyroid hormones and cardiovascular system.
Hypothyroidism is the prevailing functional disorder of the thyroid gland [5]. It is a consequence of decreased secretion of thyroid hormones or diminish activity of tissue due to thyroid gland failure or disorder of the pituitary gland or hypothalamus impede [6]. Furthermore, overt hypothyroidism is elucidated as uplifted serum thyrotropin (thyroid stimulating hormone (TSH)) concentration and serum T4 (free thyroxine) below the reference range, while subclinical hypothyroidism is described as an overhead serum TSH value accompanying a serum-free T4 within the reference range [7].
Hypothyroidism is characterized by a decrease in oxygen and substrate utilization by all the major organ systems of the body. As a result, the demand for cardiac output decrease; in addition, hypothyroidism directly alters cardiac function through changes in myocyte-specific gene expression [8].
The major cardiovascular changes that occur in hypothyroidism include a decrease in cardiac output and cardiac contractility, a reduction in heart rate, and an increase in peripheral vascular resistance (Figure 2).
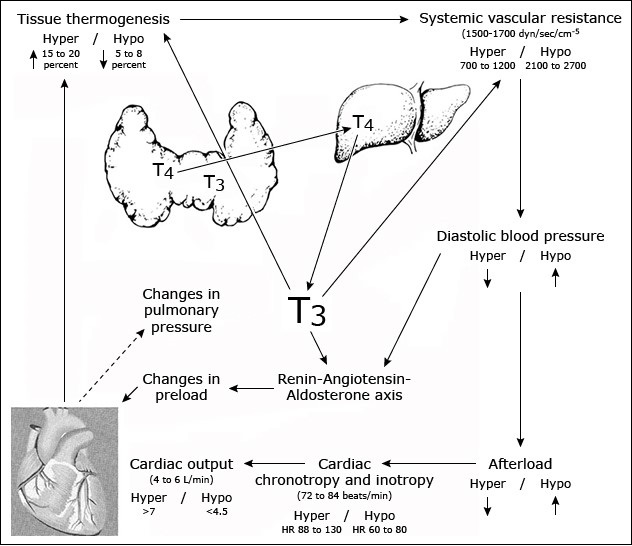 Figure 2: Cardiovascular changes in hypothyroidism.
Figure 2: Cardiovascular changes in hypothyroidism.
Thyroid diseases are incompatible with other diseases in terms of their dexterity of diagnosis, accessibility of medical treatment, and the relative visibility that even a small swelling of the thyroid offers to the treating physician. Early diagnosis and treatment remain the mainstay of management [3].
Thyroid hormones have cellular execution on almost all the tissues of the body which causes multiple organ dysfunction [9, 10]. The cardiovascular obstacles are some of the most intense and reproducible clinical findings associated with thyroid disease [11]. Furthermore, hypothyroidism is accompanied by escalated cardiovascular mortality and morbidity. The dysfunction ranges from functional systolic/diastolic dysfunction to further overt failure [12]. An earlier systematic study was done to know the prompt effects of both overt and subclinical hypothyroidism on the cardiovascular system. The identification of patients with both overt and subclinical hypothyroidism is an important individual and worldwide public health issue which can curtail accessory cardiovascular changes [13, 14]. Additionally, in primary hypothyroidism, there is an inadequacy within the thyroid gland and in secondary hypothyroidism, indirect pathologies (disorders of the hypothalamus or pituitary gland) accord to the decrease in circulating hormone levels. Moreover, overt hypothyroidism cites cases in which the serum TSH concentration is upraised and serum FT4 (free tetraiodothyronine) is below the reference range. Subclinical hypothyroidism is elucidated as serum TSH above the defined upper limit of the reference range, with a serum FT4 within the reference range [2].
Cardiac contractility
All measures of left ventricular performance are impaired in both short- and long-term hypothyroidism, leading to a reduction in cardiac output. There is also a decrease in the rate of ventricular diastolic relaxation; as a result, compliance and diastolic filling are impaired. There are also significant changes in modifiable atherosclerotic risk factors, including hypercholesterolemia, diastolic hypertension, carotid intimal media thickness, and endothelium-derived relaxing factor (EDRF) (nitric oxide), which accompany overt hypothyroidism.
The reduced ventricular performance is probably multifactorial. Possible mechanisms include increases in afterload and changes in the expression of the genes for myocardial calcium regulatory proteins. Several enzymes involved in regulating calcium fluxes in the heart are controlled by thyroid hormone, including the calcium-dependent adenosine triphosphate and phospholamban. Hypothyroidism-dependent decrease in the expression and activity of these enzymes could potentially impair systolic performance and diastolic relaxation. Beta-adrenergic receptor expression is also decreased, resulting in a blunted response to catecholamine-mediated increases in inotropy [8, 15].
Vascular resistance
The thyroid hormone relaxes vascular smooth muscle cells, thereby reducing peripheral vascular resistance. Conversely, hypothyroidism causes a decrease in the release of EDRF, which in turn promotes the contraction of these cells, thereby increasing peripheral vascular resistance. This change results in reductions in cardiac output (in part because the heart cannot increase contractility to compensate) and tissue perfusion. Tissue oxygen utilization is also decreased; thus, arteriovenous (A-V) oxygen extraction is not different from that in normal subjects [16].
Symptoms and signs of cardiovascular dysfunction are not common or prominent in patients with hypothyroidism. Those that do occur include (Table 1) [8].
- Exertional dyspnea and exercise intolerance, although these symptoms are probably due to skeletal muscle dysfunction
- Bradycardia
- Hypertension resulting from the increase in vascular resistance and the fall in EDRF
- Cardiac dysfunction with poor contractility, dilatation, or pericardial effusion
- Oedema, often non-pitting
- Pericardial effusions, which occur in approximately 25% of patients and may be quite large
| Parameter | Finding |
| Systemic vascular resistance | Increased |
| Cardiac output | Decreased |
| Blood pressure |
| Systolic | Decreased or normal |
| Diastolic | Increased or normal |
| Heart rate | Decreased or normal |
| Cardiac contractility | Decreased |
| Cardiac mass | Decreased or increased |
| Blood volume | Decreased |
| Ventricular arrhythmias | Can induce or worsen |
Table 1: Hypothyroidism and the heart.
Cardiac dysfunction
The upstroke of the pulse may be slow and the left ventricular apical impulse weak. The heart may be enlarged and the heart sounds distant. ECG may show low voltage and nonspecific ST segment and Q wave changes. Occasionally, large pericardial effusion can occur, characterized by a high protein and cholesterol content (Figure 3).
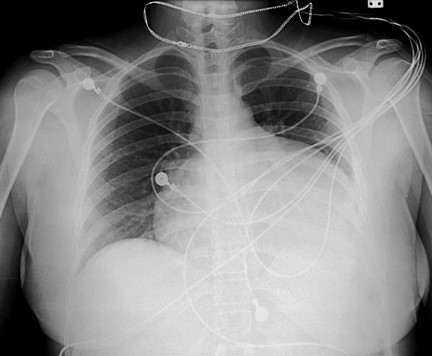 Figure 3: Pericardial effusion in a hypothyroid patient.
Figure 3: Pericardial effusion in a hypothyroid patient.
Oedema
Periorbital oedema and non-pitting oedema of the hands and feet are characteristic features of hypothyroidism. Non-pitting oedema is due to interstitial accumulation of glycols aminoglycans (hyaluronic acid and chondroitin sulphate), with associated extravascular water retention at the same time that plasma volume is decreased. Some patients have pitting oedema of the feet and legs, probably secondary to an increase in the albumin content of the interstitial fluid. Ascites, pleural, and scrotal effusions may also be present [17].
Summary
- The major cardiovascular changes that occur in hypothyroidism include a decrease in cardiac output and cardiac contractility, a reduction in heart rate, left ventricular hypertrophy, diastolic dysfunction, pericardial effusion, dilated left atrium, pulmonary artery hypertension, and an increase in peripheral vascular resistance (hypertension).
- Symptoms of cardiovascular dysfunction are not common or prominent in patients with hypothyroidism. They may include exertional dyspnoea, exercise intolerance, and oedema. Findings on physical examination may include bradycardia, hypertension, non-pitting oedema, and pleural or pericardial effusion (Table 1). Impaired cardiac muscle relaxation, decreased heart rate, and decreased stroke volume contribute to heart failure in hypothyroidism.
- Dyslipidaemia is common in hypothyroidism. The usual findings are high serum total and low-density lipoprotein (LDL) cholesterol concentrations.
The changes in cardiovascular function in hypothyroidism respond to replacement therapy with thyroxine (T4) (Figure 4).
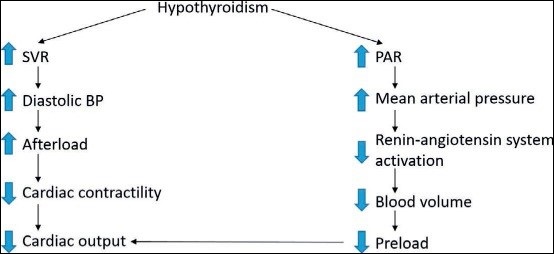 Figure 4: Hypothyroid and cardiac activity.
Figure 4: Hypothyroid and cardiac activity.
Materials and Methods
A retrospective study was carried out on 150 patients of Parul Sevashram Hospital during the period from Nov 2021 to Jan 2022 after obtaining approval from Institutional Ethics Committee. The data was collected in the Patient Profile Form (PPF) for the complete duration of therapy, the analysis made from the data was reported in predesigned forms which includes information such as patient demographic details (BP, all vitals, weight, medical and medication history, physical examination, etc.), thyroid profile and required laboratory information were performed and documented.
Univariable and multivariable logistic regression methods were used to explore risk factors associated with in-hospital. Correlations of electrocardiogram (ECG) and 2D echo upon hospital visit with disease severity and in-hospital condition were analysed. An Excel curve was used to determine the optimal condition of the heart and cardiovascular functions during the hospital visit.
- Observation was carried out to find out the scope of the study in the Parul Sevashram Hospital
- Relevant literature was reviewed
- Data collection form was designed
- Data of the patients was recorded in Patient Profile Form and analysed for the role of (Study Title After Confirmation)
Study criteria
- Age about ≥25 years
- Subjects having confirmed diagnosis of hypothyroidism
- Patients without microangioma
- Absence of ocular disease
- Pregnant, lactating women
- Mentally ill or other psychological subjects
- Subjects who are on antineoplastic medication
- Other comorbid diseases or conditions which can interfere with the study as per the investigator’s discretion
- Biochemical estimation
Physical examination, all vitals, RBS, HbA1c, hematology, thyroid profile, C-reactive protein (CRP), trop I, serum electrolyte, IL6, lipid profile, and echocardiography. Coagulation profile, renal and liver function, creatine kinase, myocardial enzymes, CRP, and procalcitonin were collected routinely on admission as and when required.
- Radiological estimation
X-ray of the chest, Doppler ultrasound, and CT pulmonary angiography were done for any patients with high clinical suspicion of pulmonary embolism (PE)/deep vein thrombosis (DVT) if required.
- Statistical analysis
The data was represented graphically in MS-Excel with median values.
Results
A total of 150 patients were included in this study where 57 were male and 93 were female, aged between 28–76 years. Patients were examined in a prospective manner and results were compared with the control group to evaluate the effect of hypothyroidism, subclinical and overt, on the cardiac status by echocardiography.
Of 150 patients studied, 51 (34%) were overt hypothyroid and 99 (66%) were found subclinical hypothyroid. Hypothyroidism was newly diagnosed more in female subjects and maximum in the age group of 35–60 years. Variables of heart structure and function were assessed by cross-sectional and Doppler echocardiography. Interventricular septum (IVS) dimensions were significantly raised in moderate subclinical and severe overt hypothyroidism (mean 0.9 +/- 0.03 and 0.9 +/- 0.2 cm). Left ventricular posterior wall (LVPW) thickness was significantly increased only in overt hypothyroidism (mean1.3 +/- 0.2).
Prehypertension (systolic) was present in 31 males and 58 females which constituted 89 (59.3%) of the total population. Stage 1 systolic hypertension is present in 50 (33.3%) and stage 2 systolic hypertension is present in 22 (14.6%). Prehypertension (diastolic) was present in 20 males and 81 females which constituted 67.3% of the total population. Similarly, Stage 1 diastolic hypertension was present in 16.6% of the study group and stage 2 diastolic hypertension was present in 7.9% of the study group. ECG findings were normal in 46% of the patients. Additionally, bradycardia was commonly seen in 44.0%, ST segment changes in 41.0%, low-voltage complexes in 37.0% of patients, and T-wave abnormality (flat/inverted) in 30% of patients. 2D echo findings were normal in 36% of patients. Diastolic dysfunction with pericardial effusion was found in 74 (49.3%) cases followed by diastolic dysfunction in 49 (32.6%), systolic dysfunction in 15 (10.0%), and increased interventricular septum spectrum thickness in 12 (8.6%) patients. The majority of the diastolic dysfunction was mild dysfunction associated with females. No cases were found to have severe diastolic dysfunctions.
Cardiovascular symptoms were seen in 106 (70.66%) patients, which include effort intolerance in 89 (59.3%) patients, chest pain in 65 (43.3%) patients, breathlessness in 58 (38.6%) patients, and palpitation in 86 (57.3%) patients of each. In this study, low voltage complexes in ECG were found in 32 (21.0%) patients, of which 11 (7.3%) were male and 21 (14 %) were female. Similarly, 19% of males had T-inversion in V3–V6 leads. However, RVW and LVID showed no definite pattern of change. Pericardial effusion and diastolic dysfunction were seen in 74 cases only in overt hypothyroidism.
Discussion
On the basis of a case history, the clinical and para-clinical manifestations of hypothyroidism are reviewed. Exertion dyspnea without signs of cardiac insufficiency occurs frequently. The minute and stroke volume and heart rate are reduced. This study included 150 patients diagnosed with hypothyroidism. The patient’s age range was 28–77 years in the study. Most patients belonged to the age group of 30–68 years. Overall, there was a female preponderance over the age of 30–68 years. The female population constituted about 59.3% of the total which is an approximate female:male ratio of 2:1.
The blood pressure may rise (reversible) and hypertension may occur. The function of the left ventricle is reversibly reduced. X-ray of the thorax may reveal massive relatively asymptomatic pleural effusion and cardiomegaly. Pericardial exudate occurs frequently and is demonstrated best by echocardiography. The plasma concentrations of several different enzymes (including creatine kinase (CK), CK-MB, and LDH) may be raised in myxedema. The reason for this is perhaps compromised membrane function in the skeletal muscle cells.
On general examination of patients during study and follow-up, we found the common symptoms were weight gain, 61.5% in males and 38.5% in females; aches and pain, 63.1% in males and 38.9% in females; menorrhagia, 47% in females; intolerance to cold was around 41.1% in males and 28.7% in females; swelling of limbs in 25.0% males and 30.8% females; similarly, puffiness of face in 38.5% males and 29.3% females; hair loss was found in 17.0% females and 22.5% males. Chest pain was found in 43.3% of patients and breathlessness was found in 38.6% of subjects. All these findings were similar compared to the other published studies by Watanakunakorn et al. [18], Kumar [19], Pyarsabadi et al. [20], and Reavey et al. [21].
On physical examination, the general signs included body mass index > 24 kg/m2 in 49.6% of patients, thyromegaly seen in a few patients were excluded and referred to the surgery department for further course of treatment, and lower limb oedema in 37.3% males and 67.7% females. In ECG changes, bradycardia was present in 44%, low-voltage complexes in 32%, followed by T-wave (flat/inverted) changes in 19.3%, and ST segment changes in 36.0%. In 2D echo, the findings and changes were that left ventricular diastolic dysfunction was abnormal with pericardial effusion in 49.3%, LWH in 35.4%, RWMA in 16.4%, and ejection fraction (< 60%) in 38.5% patients. This finding is consistent with other studies by Varma et al. [22] that showed the prevalence of effusion to be 22.75%. Rawat et al. [13], studied that pericardial effusion is reported to occur in 30–70% of patients with hypothyroidism. Furthermore, a few published studies by Chahine et al. [23], Coetzee et al. [24], show a relatively low degree of pericardial effusion, which may be due to the selection of newly diagnosed hypothyroid. In our study, the major associated manifestations were dyslipidaemia, systolic and diastolic hypertension, ECG changes, and echo changes. There was a statistically significant interconnection between degrees of hypothyroidism and all these cardiodyslipidemic manifestations. We suggest that all these findings need to be performed in a larger group for further evaluation.
Conclusion
Hypothyroidism, both subclinical and overt, is associated with cardiovascular alteration, both structural and functional. IVS and LVPW thickness are markedly affected, and there is an impairment in left ventricular function in diastole. Furthermore, hypothyroidism is more common in females, between the age group of 20–50 years. The majority of the patients have cardiovascular changes such as ECG abnormalities, pericardial effusion, diastolic dysfunction, and diastolic hypertension. We strongly suggest early detection and initiation of hormone replacement therapy can minimize the associated cardiovascular changes.
Limitation
The only limitation of this study was a single center study. Indeed, it needs a multicenter study to evaluate the same. Our study used a comorbid subject that was probably interfering with study results, long-standing inflammatory status, comorbid disease condition, and other medical conditions in the human body which might interfere with the study result.
Acknowledgments
We are sincerely thankful to all the participants, and management of Parul Sevashram Hospital, Vadodara for their grateful cooperation. We are also grateful to the teaching and non-teaching staff of the Department of Medicine, Cardiology, ICU, and Biochemistry for their valuable work and time.
References
- Razvi S, Jabbar A, Pingitore A, et al. Thyroid Hormones and Cardiovascular Function and Diseases. J Am Coll Cardiol. 2018;71(16):1781-96.
- Khatri P, Sapkota S, Neupane A, et al. A Study of Echocardiographic Changes in Patients with Newly Diagnosed Primary Hypothyroidism: A Cross-sectional Study. Europasian Journal of Medical Sciences. 2021;3(2):23-29.
- Unnikrishnan AG, Menon UV. Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab. 2011;15(Suppl2):S78-81.
- General Information/Press Room. American Thyroid Association.
- Boelaert K, Franklyn JA. Thyroid hormone in health and disease. J Endocrinol 2005;187(1):1-5.
- Kronenberg HM, Melmed S, Polonsky KS, et al. Williams Textbook of Endocrinology. 11th ed. Philadelphia: Saunders Company; 2007. Chapter 10: Thyroid Physiology and Diagnostic Evaluation of Patients with Thyroid Disorders, Chapter 12: Hypothyroidism and Thyroiditis; p. 377-405.
- Calsolaro V, Niccolai F, Pasqualetti G, et al. Overt and Subclinical Hypothyroidism in the Elderly: When to Treat? Front Endocrinol (Lausanne). 2019;10:177.
- Klein I, Danzi S. Thyroid Disease and the Heart. Circulation 2007;116(15):1725-35.
- Braunwald E, Zipes DP, Libby P, et al. The Heart Disease: A Text Book of Cardio Vascular Medicine. 9th ed. Philadelphia: Saunders; 2012. The heart in endocrine disorders; p. 183-340.
- Guyton AC, Hall JE. Textbook of Medical Physiology, 11th ed. Mississippi: Saunders; 2017. Chapter 76: Thyroid Metabolic Hormones; 931-43.
- Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363(9411):793-803.
- Klein I, Ojamaa K. Thyroid Hormone and the Cardiovascular System. N Engl J Med. 2001;344(7):501-09.
- Rawat B, Satyal A. An Echocardiographic Study of Cardiac Changes in Hypothyroidism and the Response to Treatment. Kathmandu Univ Med J (KUMJ). 2004;2(3):182-87.
- Fazio S, Palmieri EA, Lombardi G, et al. Effects of Thyroid Hormone on the Cardiovascular System. Recent Prog Horm Res. 2004;59:31-50.
- Libby P, Bonow Robert, Mann D, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Philadelphia: Elsevier; 2021. Chapter 96: Endocrine disorders and cardiovascular disease; p. 1807.
- Rothberger GD, Gadhvi S, Michelakis N, et al. Usefulness of Serum Triiodothyronine (T3) to Predict Outcomes in Patients Hospitalized With Acute Heart Failure. Am J Cardiol. 2017;119(4):599-603.
- Kabadi UM, Kumar SP. Pericardial Effusion in Primary Hypothyroidism. Am Heart J. 1990;120(6 Pt 1):1393-95.
- Watanakunakorn C, Hodges RE, Evans TC. Myxedema: A Study of 400 Cases. Arch Intern Med. 1965;116(2):183-90.
- Kumar P. A Study of Cardiovascular Changes in Newly Detected Hypothyroid Patients. 2020.
- Pyarsabadi P, Saluja M, Chittora S, et al. An Unusual Case of Pericardial Tamponade in Primary Hypothyroidism. Int J Adv Med. 2017;4(2):581-84.
- Reavey JJ, Walker C, Murray AA, et al. Obesity is Associated with Heavy Menstruation that May be Due to Delayed Endometrial Repair. J Endocrinol. 2021;249(2):71-82.
- Varma R, Jain AK, Ghose T. Heart in hypothyroidism–an echocardiographic study. J Assoc Physicians India. 1996;44(6):390-92.
- Chahine J, Ala CK, Gentry JL, et al. Pericardial Diseases in Patients with Hypothyroidism. Heart. 2019;105(13):1027-33.
- Coetzee A, Kyriakakis C, Greyling C, et al. Cardiac Tamponade Due to Hypothyroidism: a Case Cluster report. Journal of Endocrinology, Metabolism and Diabetes in South Africa. 2016;21(2):16-19.

 Figure 1: Interaction between thyroid hormones and cardiovascular system.
Figure 1: Interaction between thyroid hormones and cardiovascular system. Figure 2: Cardiovascular changes in hypothyroidism.
Figure 2: Cardiovascular changes in hypothyroidism.
