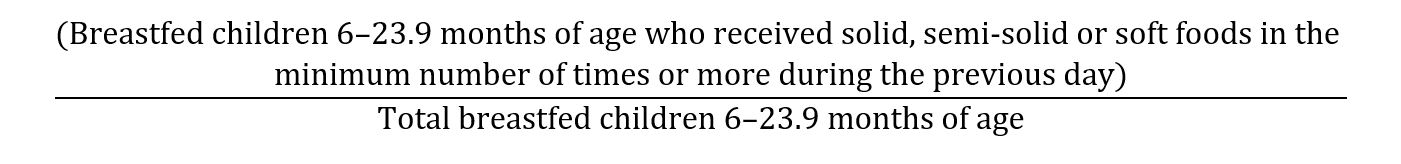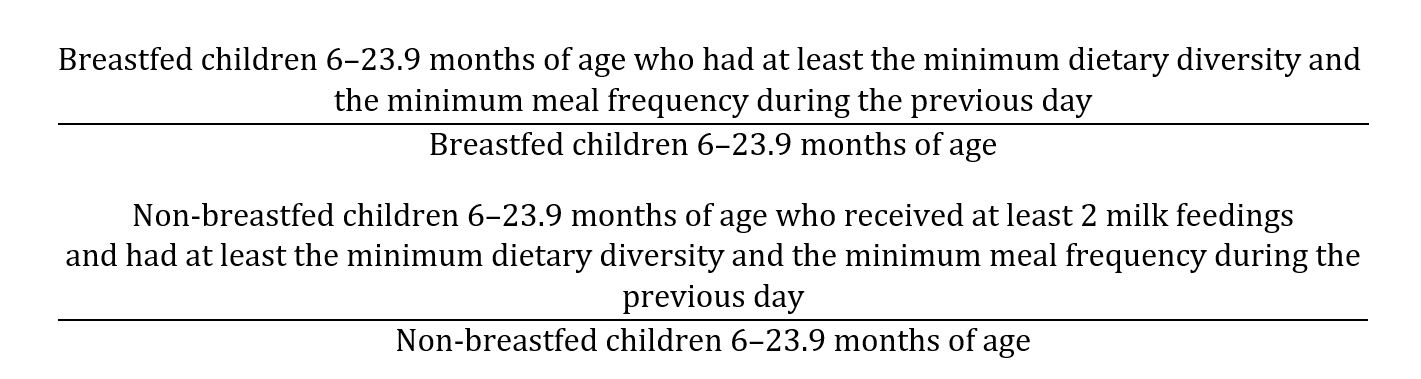Abstract
Introduction: Infant and young child feeding (IYCF) practices have substantial outcomes for the growth, development, and survival of infants and children during the first two years of age and throughout their lifetime. The study aimed to assess the dietary intake and nutritional status of children (0–23 months) registered for care in Hasiya Bayero Pediatric Hospital Kano.
Methodology: A cross-sectional study was conducted among 125 randomly selected caregiver-child pairs accessing care in the hospital. A validated semi-structured questionnaire was used to collect information on socio-demographic and dietary intake information from the respondents. Anthropometric indices of weight for age, length for age, and weight for length measurements were used to assess the nutritional status of children.
Result: Female caregivers were the most participants in this study 122 (98.2%). For marital status, almost all 124 (99.4%) of the mothers were married and only 1 each of the mothers reported to be single and being widowed. About 76 (60.8%) of the study population attended secondary education. The major occupation of the caregivers is trading in 92.4%. Dietary intake consists mainly of legumes in 30.0% and cereals in 56.5%. Only 39.0% portion of the population met the minimum World Health Organization (WHO) recommendation of dietary diversity and over 79.8% of the caregivers were still breastfeeding during the period of the study, 18% of them initiated breastfeeding within 1 h of birth and only 16.2% exclusively breastfed their children; majority 70.6% of the caregivers breastfed on demand while with a small proportion of 26.1% and 3.3% responded to breastfeeding when convenient and breastfeeding at a scheduled time respectively.
Conclusion: The study revealed that the nutritional status of the children was poor. Up to 60 (51.2%), 98 (88.3%), and 74 (65.1%) of the infants were wasted, underweight, and stunted, respectively. The study also revealed that the nutritional status of the children (WLZ, WAZ, and LAZ) was significantly related to the dietary diversity at p = 0.002, p = 0.001, and p = 0.019, respectively. This study revealed poor IYCF practices in Hasiya Bayero Pediatric Hospital Kano. Therefore, more attention needs to be paid to the specific behaviors surrounding feeding practices and other constraints to children accessing care in Hasiya Bayero Pediatric Hospital Kano.
Keywords
dietary intake, nutritional status, infant and children, Kano, Nigeria
Abbreviations
WHO: World Health Organization; SAM: severe acute malnutrition; IYCF: infant and young child feeding; NFHS: National Family Health Survey; LGA: local government area; NDHS: National Demographic Health Survey; MDD: minimum dietary diversity; MMF: minimum meal frequency; NFHS-4: National Family Health Survey-4
Introduction
Infant feeding practices in Nigeria have been a topic of concern due to their impact on child health and development. According to a survey conducted by the Nigeria Demographic and Health Survey [1], exclusive breastfeeding rates remain low at 29% meaning that over 70% of infants in Nigeria are denied the benefits of breast milk in their formative age, while 35% of infants under six months receive complementary foods along with breast milk, and 36% are not breastfed at all [2]. Several factors contribute to poor infant feeding practices in Nigeria. Only 9% of organizations have a workplace breastfeeding policy, indicating that mothers lack the enabling environment to optimally breastfeed their babies. Other factors include inadequate knowledge of breastfeeding, traditional beliefs, cultural practices, and poverty. Mothers in rural areas are less likely to breastfeed exclusively, and those who work outside the home face challenges in sustaining exclusive breastfeeding due to a lack of maternity leave and facilities for expressing and storing breast milk [2]. The promotion of optimal infant feeding practices in Nigeria requires a multi-faceted approach that involves the education of mothers, families, and communities, the support of health care providers and policymakers, and the provision of resources to enable mothers to breastfeed and provide appropriate complementary foods.
Dietary intake refers to the long-term average daily intake of nutrients or foods [3]. Nutritional research has expanded to consider whole diets in addition to individual nutrients and foods [4]. Whole diet measures can be based on food intake assessed against a pre-determined index, or empirically, whereby variables are reduced into a small number of components through statistical manipulation [4]. For example, factor analysis is used to derive a dietary pattern score reflecting foods that correlate with each other [6]. Although early life is a significant period when dietary preferences and habits are first established, laying the foundation of adult eating habits [7]. As dietary patterns are likely to be age-specific, understanding early-life dietary patterns, their determinants and their influence on later health is important for developing strategies to improve nutrition in early childhood [7].
Nutritional status is defined as the evident state of an individual health condition in relation to their intake and utilization of nutrients [8]. The nutritional status of children is important as it determines their health, physical growth and development, academic performance, and progress in life [9]. Moreover, good nutrition has been reported to be the cornerstone for survival, health, and development in the current and succeeding generations [10]. Therefore, the physical, mental, social, and nutritional status of children, as well as other characteristics related to malnutrition should be assessed routinely to monitor and evaluate malnutrition, in order to provide information for policymakers to make a decision [8].
Malnutrition is a condition that results from undereating and overeating a diet which may result in metabolic disorders such that the diet can cause adverse health effects [8]. It may involve calories, protein, carbohydrates, vitamins, or minerals [11]. Therefore, malnutrition is a serious medical condition marked by a deficiency of energy, essential proteins, fats, vitamins, and minerals in a diet [12]. Over 10 million children aged less than two years (under two children) die annually from preventable and treatable illnesses and almost all these deaths occur in poor countries [13]. Malnutrition caused more than one-third of all deaths of under two children [14]. Currently, 195 million under two children are affected by malnutrition; 90% of them live in sub-Saharan Africa and South Asia. At least 20 million children suffer from severe acute malnutrition (SAM), and another 157 million are undernourished [8]. Malnutrition is the most recognizable and perhaps most untoward consequence of poverty in children [5]. In sub-Saharan Africa and most developing countries, extreme urban poverty is concentrated in temporary or informal squatter settlements [8].
The infant and young child feeding (IYCF) program is a key priority information program of the government to enhance the competencies and build the capacity of health workers and caregivers of under two children for the survival, growth, and development of children in the first 1000 days of life [15]. IYCF practices are crucial for nutritional status, growth, development, health, and ultimately the survival of infants and young children [16, 17]. Worldwide, suboptimal breastfeeding still accounts for the deaths of 1.4 million children aged less than five years (under five mortality) [12]. The timely introduction of complementary feeding can prevent almost 6% of under-five mortality [18]. It was estimated that, if 90% of infants are covered with a package of interventions to protect, promote, and support the optimal IYCF practices, almost one-fifth of overall under-five mortalities can be averted [18]. The poor complementary feeding practices mean that many children continue to be vulnerable to irreversible outcomes of stunting, poor cognitive development, and a significantly increased risk of infectious diseases, such as diarrhea and acute respiratory infection [17, 19, 20]. The World Health Organization (WHO) recommends exclusive breastfeeding for the first six months of life with early initiation and continuation of breastfeeding for two years or more together with nutritionally adequate, safe, and age-appropriate complementary feeding starting at six months [21].
The National Family Health Survey (NFHS) has provided useful national and state-level information on the IYCF practices [22]. Available data showed a gross inter-state variation. However, the NFHS was not designed to provide district-level [23].
To date, the information has been adopted, to varying degrees, in nearly every state in Nigeria [14]. All partners agreed that for the evaluation, the package would be implemented at scale in one local government area (LGA) in Kano State that had not previously benefited from any IYCF-related program [11]. The prevalence of nutritional status of under five children in Kano State is unacceptably low based on; stunting (2.2%), wasting (0.3%), and underweight (1.5%) [1]. To the best of our knowledge, there is no report on the nutritional status of the catchment area of the study location despite many governmental and non-governmental interventions over the years [14]. Therefore, there is a need to generate information on the dietary intake and nutritional status of children (0–23 months) in IYCF practices in Hasiya Bayero Pediatric Hospital Kano. This study is designed to recognize the gap in evidence-based guidance, tools, and other resources for training and counseling at the community level, UNICEF and its partners officially launched the generic C-IYCF Counselling Package [14].
Materials and Methods
Kano State (Hausa: Jihar Kano, Fula: Leydi Kano), is one of the 36 states of Nigeria, located in the northern region of the country [28]. According to the national census done in 2006, Kano State is the most populous state in Nigeria [29]. The recent official estimates taken in 2016 by the National Bureau of Statistics found that Kano State was still the largest state by population in Nigeria [30, 31]. Created in 1967 out of the former Northern Region, Kano State borders Katsina State to the northwest, Jigawa State to the northeast, Bauchi State to the southeast, and Kaduna State to the southwest [32]. The state’s capital and largest city is the city of Kano, the second most populous city in Nigeria after Lagos [32]. Modern-day Kano State was the site of a number of prior kingdoms and empires, including the Kingdom of Kano, which was centered in Dalla Hill and existed from prior to 1000 AD to 1349 [33, 34]. In 1349, the Sultanate of Kano was established with Yaji I as its first Sultan [35]. In the 15th century, Kurmi Market was opened, which helped Kano become a center of commercial activity in Hausaland [25]. The market remains open in the 21st century and its historic importance is reflected in the state’s nickname, the Centre of Commerce [35]. During the 16th and 17th centuries, the Sultanate of Kano established itself as the most powerful of the Hausa Kingdoms [26]. In 1903, the British Empire conquered the Kano Emirate, incorporating its region into the Northern Nigeria Protectorate [27]. The major ethnic groups in pre-colonial Kano City were the Hausa, Fulani, Beriberi (Kanuri), Tuareg, Arab, Nupe, and some tribes from southern Nigeria. Most people in Kano city have come to use the Hausa language as a first language and some have accepted Hausa as an ethnic identification [36].
Since independence, Kano State has developed a diverse economy, establishing itself as a center for industry [37], agriculture [38], and Islamic banking [39]. The Hausa and Fulani make up the majority of Kano State’s population [40]. The Hausa language is the dominant language in the state, as it is in most of Northern Nigeria [41, 42]. Challenges faced by Kano State in the 21st century include attacks by the Islamist terrorist group Boko Haram [43–45], inter-religious violence [41, 42], and extreme poverty [46]. A Muslim-majority state, Kano State is one of the 12 states in Nigeria to operate under Sharia law within the legal framework of the Nigerian Constitution [43].
Kano state consists of over 499 health facilities, including public/private ownership and primary/secondary/tertiary healthcare facilities [47]. Kano State has 6 universities and 24 colleges of education, including polytechnic and health technologies [48]. Traditionally, the people of Kano used various means to provide water for domestic consumption use in cottage industries and other purposes. Some major sources of water include wells, dams, rainfall/rainwater, and rivers [49].
It was reported in a study carried out by Umar et al. [50] in Fagge Area of Kano State that majority of waste segregation is 98.4%, sharps waste collection using bare hands is 62.2%, while 32.5% of wastes are transported using wheelbarrow within the hospital and 44.0% using carts by Yaro Boys for offsite transportation, 76.6% are stored for 12 h, while treatment of infectious waste found to be 0.0%, and 74.2% health facilities practiced land disposal method.
Pretesting of instruments
The instruments to be used for this study were pre-tested in Aminu Kano Teaching Hospital before the commencement of the research work to ensure accuracy and avoid errors during the measurement of research parameters.
Study of the instruments
The equipment used for this study included an electronic digital weighing balance, sample MUAC tape, infantometer/stadiometer, validated semi-structured questionnaire, writing materials, test tube. All the instruments used for this study were carefully and properly studied before the commencement of the research work to ensure the proper handling and operation to avoid errors.
Sample size calculation
The sample size was calculated using (conventional) Fischer’s formula as described by WHO [24]
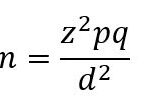 Where:
Where:
n = the desired sample size
z = the standard normal deviate usually set at 1.96 (which corresponds to the 95% confidence level)
p = the prevalence of malnutrition in the target population (9.2% prevalence of wasting in the National Demographic Health Survey (NDHS) conducted in 2018) [1]
q = 1-p
d = absolute precision or accuracy, normally set at 0.05.
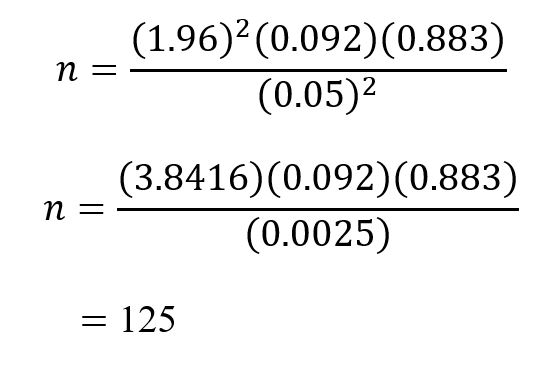 Sampling technique
Sampling technique
A multiple-stage sampling method was used which included a convenience sampling method and a simple random sampling technique. Convenience sampling was used to determine the study population based on the number of caregivers/children pairs at the hospital facility during the period of the study. A simple random sampling technique was used to determine the equal sample size by choosing the caregivers/children accessing care in the hospital facility using a random number table from a complete list of caregivers/children accessing care in Hasiya Bayero Pediatric Hospital Kano. 125 caregivers/children accessing care in Hasiya Bayero Pediatric Hospital Kano were selected using convenience sampling and simple random sampling techniques.
Training of research assistants
Five research assistants among the health workers in Hasiya Bayero Pediatric Hospital were trained on how to carry out data collection by interviewing the targeted population and how to measure anthropometric parameters.
Field data collection
This study was a hospital-based descriptive cross-sectional survey. Socio-demographic characteristics of the children were assessed using a validated semi-structured questionnaire which was administered through interviews conducted by trained interviewers. The anthropometric indices (weight/length for age, length for age, weight for age, and MAUC) of children (0–23 months) were taken to assess nutritional status. The dietary diversity scores were used to assess the nutrient intake of the children. The dietary pattern of breast and complementary feeding practices was later compared to the national guideline as standard.
Food diversity of the children was determined to get the type of foods and beverages, and also to know the breastfeeding and complementary feeding practices adopted within the previous day and as the child is growing using a semi-structured questionnaire.
The indicator (minimum meal frequency) was calculated from the following fraction [35]
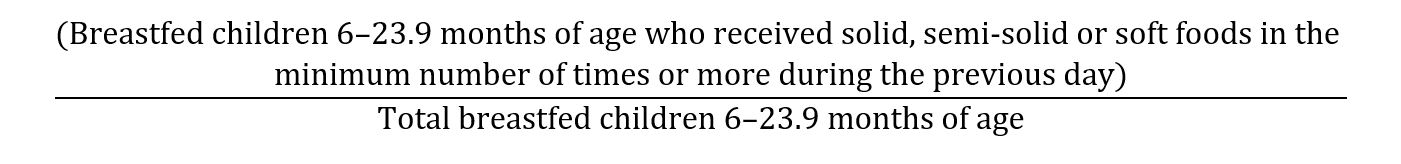
- Minimum dietary diversity
The 8 food groups used for tabulation of this indicator (minimum dietary diversity) are grains, roots and tubers, legumes and nuts, milk and products (breast milk, yogurt, and cheese), flesh foods (meat, fish, poultry, and liver/organ meats), eggs, vitamin-A rich fruits and vegetables, and other fruits and vegetables.
This composite indicator (minimum acceptable diet) will be calculated from the following two fractions [35]
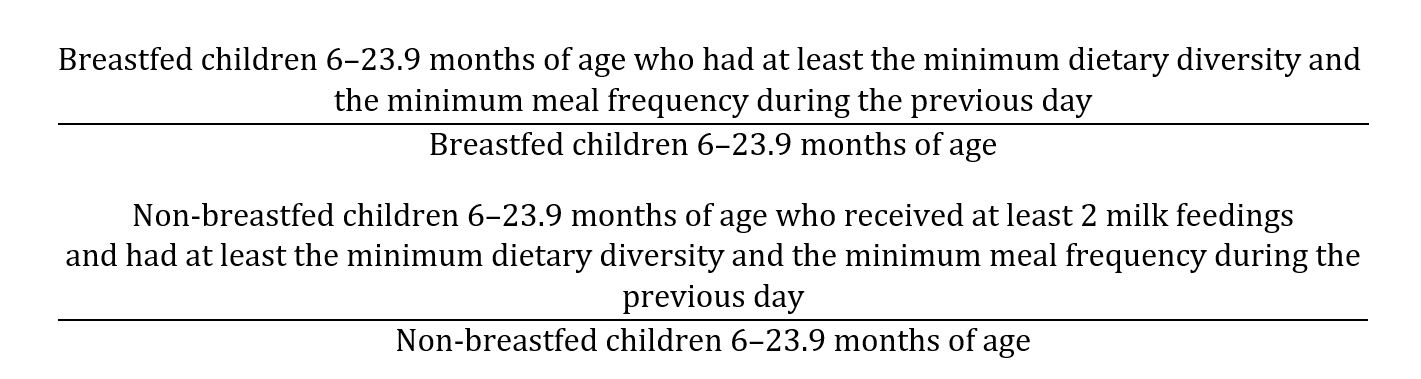
Data analysis
Data obtained were analyzed using SPSS version 20. Data were presented as mean ± SD. Descriptive statistics were used to summarize data obtained on socio-demographic characteristics, bivariate and multivariate and chi-square tests were used for categorical variables, and the software was used to analyze anthropometry indices.
IYCF practices questionnaire were coded and analyzed, and frequencies and percentages for various responses were generated to describe caregivers’ practices using SPSS version 20.
Results
The survey was conducted among 125 caregivers of the children accessing care in Hasiya Bayero Pediatric Hospital Kano (Tables 1 and 2, Figures 1 and 2, and Tables 3–11).
| Variables | | Frequency | Percentage (%) |
| Sex of caregiver | Male | 3 | 1.8 |
| Female | 122 | 98.2 |
| Age of caregiver | Below 20 years | 5 | 3.5 |
| 21–25 years | 34 | 27.6 |
| 26–30 years | 32 | 25.3 |
| 31–40 years | 45 | 35.3 |
| Above 40 years | 9 | 8.3 |
| The mean age of caregivers | 31.0 ± 8.0 years | |
| Sex of child | Male | 74 | 57.9 |
| Female | 51 | 42.1 |
| Age of Child | 0–5 months | 24 | 21.2 |
| 6–12 months | 82 | 55.3 |
| 13–20 months | 14 | 14.7 |
| 21–23 months | 5 | 8.8 |
| The mean age of children | 11.0 ± 6.0 months | |
| Marital status of the caregiver | Single | 0 | 0.0 |
| Married | 124 | 99.4 |
| Divorce | 0 | 0.0 |
| Widow | 1 | 0.6 |
| Educational status of the caregiver | Primary | 32 | 27.9 |
| Secondary | 76 | 60.5 |
| Vocational | 7 | 3.8 |
| Technical | 0 | 0.0 |
| Post-Secondary | 0 | 0.0 |
| Tertiary | 10 | 7.8 |
| Socio-economic status of the caregiver | Low | 58 | 52.8 |
| Middle | 48 | 43.7 |
| High | 5 | 3.5 |
| Occupation of caregiver | Farmer | 19 | 11.0 |
| Business | 100 | 85.4 |
| Civil servant | 6 | 3.6 |
| Average monthly earnings | N10,00 and below | 112 | 92.4 |
| 11,000–20,000 Naira | 6 | 3.5 |
| 21,000–50,000 Naira | 4 | 2.4 |
| 51,000–100,00 Naira | 3 | 1.7 |
| Above 100,000 Naira | 0 | 0.0 |
| Mean | 9,500 ± 1.000 Naira | |
| Number of people in a household | 1–5 members | 43 | 32.9 |
| 6–10 members | 53 | 45.9 |
| 11–15 members | 16 | 13.5 |
| 16–20 members | 6 | 3.6 |
| > 20 members | 7 | 4.1 |
| Mean HH members | 8.0 ± 5.0 members | |
Table 1: Socio-demographic characteristics of caregivers of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
| Food groups | 1 or less times/week | 1–3 times/week | 4–6 times/week | 7 or more times/week |
| n (%) | n (%) | n (%) | n (%) |
| Meat, fish, and their products | 70 (58.8) | 35 (29.4) | 13 (7.6) | 7 (4.2) |
| Milk, yogurt, dairy, and their products | 61 (62.8) | 37 (21.6) | 20 (11.4) | 7 (4.2) |
| Legumes, nuts, and their Products | 9 (5.3) | 24 (17.6) | 50 (47.1) | 42 (30.0) |
| Eggs | 75 (62.1) | 33 (25.4) | 14 (10.7) | 3 (1.8) |
| Fruits | 70 (56.3) | 27 (21.6) | 18 (14.5) | 10 (7.6) |
| Cereal, grains, and their products | 6 (4.7) | 9 (7.1) | 39 (31.7) | 71 (56.5) |
| Vegetables and their products | 71 (56.5) | 30 (27.1) | 15 (11.1) | 9 (5.3) |
| Sweets and sugars | 80 (66.4) | 28 (21.2) | 14 (10.0) | 3 (2.4) |
| Beverages, tea and coffee | 77 (63.5) | 28 (21.2) | 15 (10.6) | 5 (4.7) |
Table 2: Frequency of food consumption of the children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
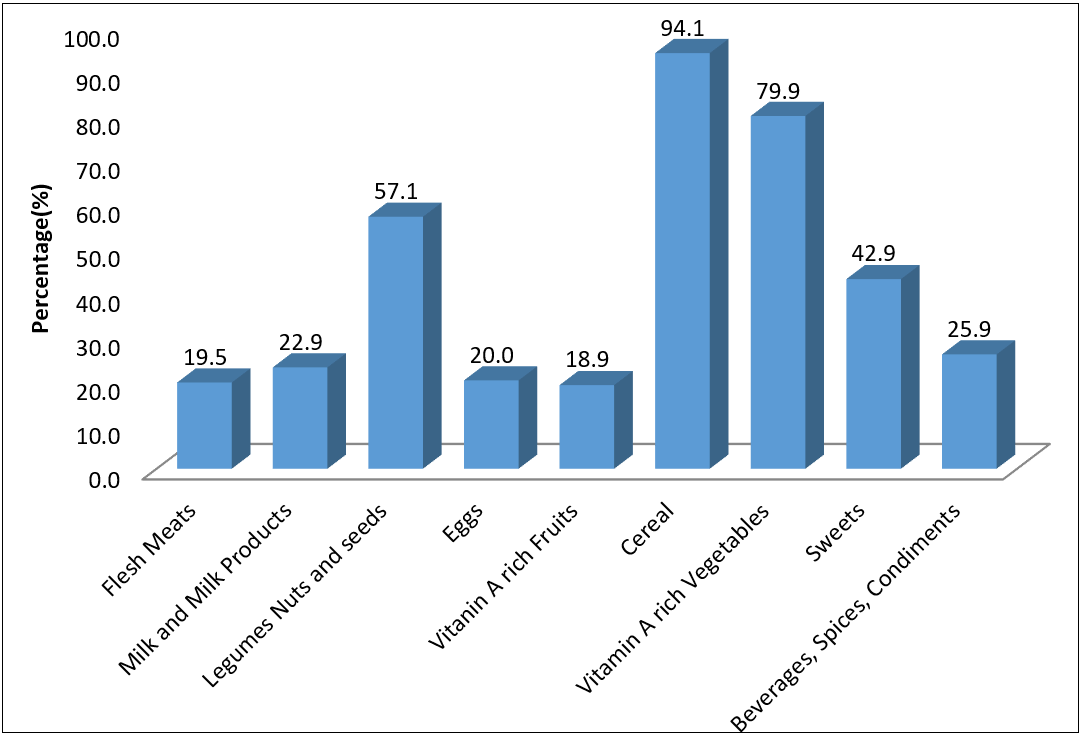 Figure 1: Food consumed by the children.
Figure 1: Food consumed by the children.
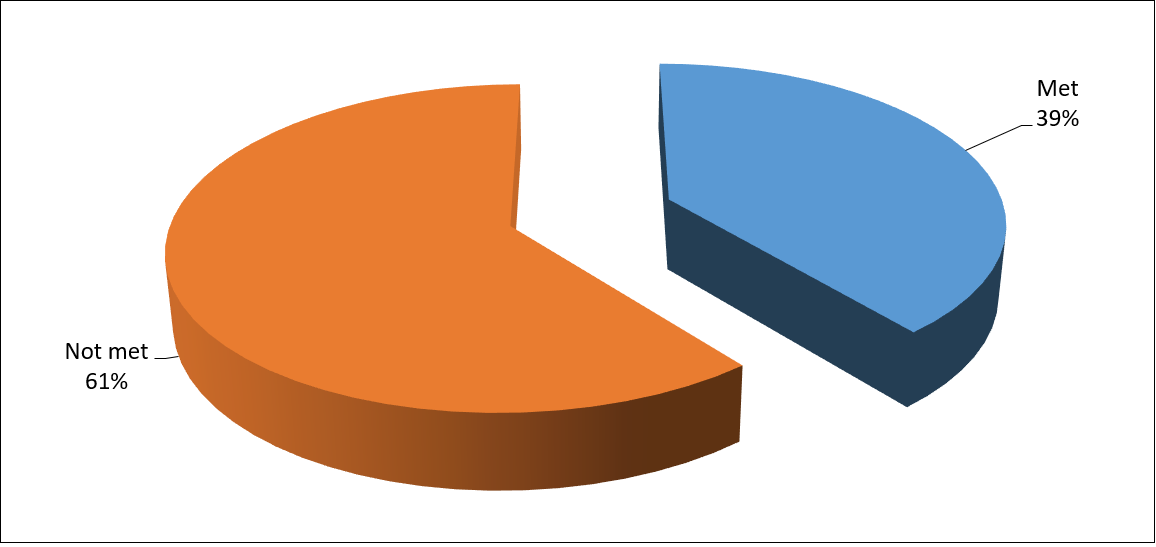 Figure 2: Minimum dietary diversity of the children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
Figure 2: Minimum dietary diversity of the children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
| Variables | | Dietary diversity |
| Met (%) | Not Met (%) | p-value |
| Sex of caregiver | Male | 50.0 | 50.0 | 0.130 |
| Female | 38.8 | 61.2 | |
| Age of caregiver | Below 20 years | 33.3 | 66.7 | 0.117 |
| 21–25 years | 42.6 | 57.4 | |
| 26–30 years | 39.5 | 60.5 | |
| 31–40 years | 37.5 | 62.5 | |
| Above 40 years | 32.1 | 67.9 | |
| Sex of child | Male | 38.6 | 61.4 | 0.683 |
| Female | 38.1 | 61.9 | |
| Age of child | 0–5 months | 34.7 | 65.3 | |
| 6–12 months | 39.4 | 60.6 | |
| 13–20 months | 40.0 | 60.0 | |
| 21–23 months | 43.3 | 56.7 | |
| Marital stataus of caregiver | Single | 0.0 | 0.0 | 0.981 |
| Married | 38.9 | 61.1 | |
| Divorce | 0.0 | 0.0 | |
| Widow | 0.0 | 100.0 | |
| Educational status of caregiver | Primary | 32.0 | 68.0 | 0.049* |
| Secondary | 40.6 | 59.4 | |
| Vocational | 40.0 | 60.0 | |
| Technical | 0.0 | 0.0 | |
| Post-secondary | 0.0 | 0.0 | |
| Tertiary | 46.2 | 53.8 | |
| Socio economic status of caregiver | Low | 35.9 | 64.1 | 0.013* |
| Middle | 40.0 | 60.0 | |
| High | 50.0 | 50.0 | |
| Occupation of caregiver | Farmer | 36.1 | 63.9 | 0.432 |
| Business | 39.6 | 60.4 | |
| Civil servant | 41.7 | 58.3 | |
| Average monthly earnings | N10,00 and below | 16.7 | 83.3 | 0.001* |
| 11,000–20,000 Naira | 33.3 | 66.7 | |
| 21,000–50,000 Naira | 25.0 | 75.0 | |
| 51,000–100,00 Naira | 39.8 | 60.2 | |
| Above 100,000 Naira | 0.0 | 0.0 | |
| Number of people in a household | 1–5 members | 60.7 | 39.3 | 0.024* |
| 6–10 members | 37.8 | 62.2 | |
| 11–15 members | 43.5 | 56.5 | |
| 16–20 members | 0.0 | 100.0 | |
| > 20 members | 21.4 | 78.6 | |
Table 3: Associations between socio-demographic characteristics and diet of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. *Chi-square is significant at ≤0.05.
| Anthropometry indices | |
| | Frequency | % |
| WLZ | Normal (<-l Z-scores>-2 Z-scores) | 55 | 48.8 |
| Moderate Wasting (>-3 Z-scores<-2 Z-scores) | 60 | 46.5 |
| Severe Wasting (<-3 Z-scores) | 10 | 4.7 |
| WAZ | Normal (<-l Z-scores>-2 Z-scores) | 20 | 11.7 |
| Moderate Underweight (>-3 Z-scores<-2 Z-scores) | 98 | 84.9 |
| Severe Underweight (<-3 Z-scores) | 7 | 3.4 |
| LAZ | Normal (<-l Z-scores>-2 Z-scores) | 43 | 35.9 |
| Moderate Stunting (>-3 Z-scores<-2 Z-scores) | 74 | 58.2 |
| Severe Stunting (<-3 Z-scores) | 8 | 5.9 |
Table 4: Nutritional status of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. MUAC: mid-upper arm circumference; WLZ: weight for length; WAZ: weight for age; LAZ: length for age.
| Nutritional status | Minimum diversity met | Minimum diversity not met | Chi-square test |
| % | % | p-value |
| WFA |
| Normal | 77.8 | 22.2 | 0.001* |
| Mild underweight | 26.7 | 73.3 | |
| Moderate underweight | 32.0 | 68.0 | |
| Severe underweight | 8.8 | 91.3 | |
| LFA |
| Normal | 71.0 | 29.0 | 0.019* |
| Mild stunting | 10.0 | 90.0 | |
| Moderate stunting | 23.1 | 76.9 | |
| Severe stunting | 14.3 | 85.7 | |
| WFL |
| Normal | 70.5 | 29.5 | 0.002* |
| Mild wasting | 8.3 | 91.7 | |
| Moderate wasting | 29.5 | 70.5 | |
| Severe wasting | 0.0 | 100.0 | |
Table 5: Association between dietary diversity and nutritional status (WFA, LFA, and WFl) of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. *Chi-square is significant at ≤0.05.
| Variables | WFA | HFA | WFH |
| R | p-value | R | p-value | R | p-value |
| Sex of caregiver | -0.078 | 0.320 | -.158* | 0.041 | -0.011 | 0.888 |
| Age of caregiver | .915** | 0.000 | .155* | 0.044 | .362** | 0.000 |
| Sex of child | -0.121 | 0.131 | -0.056 | 0.482 | -0.04 | 0.613 |
| Age of child | 0.081 | 0.303 | .946** | 0.000 | 0.027 | 0.731 |
| Marital status of the caregiver | 0.13 | 0.105 | 0.007 | 0.928 | -0.034 | 0.666 |
| Educational status of the caregiver | -.216** | 0.006 | 0.029 | 0.711 | -.165* | 0.033 |
| Socio-economic status of caregiver | 0.074 | 0.360 | 0.082 | 0.304 | -0.034 | 0.673 |
| Occupation of caregiver | 0.003 | 0.967 | 0.151 | 0.054 | -0.008 | 0.920 |
| Average monthly earnings | 0.058 | 0.457 | 0.029 | 0.709 | -0.001 | 0.985 |
| Number of people | .445** | 0.000 | 0.076 | 0.324 | .903** | 0.000 |
| Dietary diversity | -0.059 | 0.451 | 0.14 | 0.068 | 0.022 | 0.778 |
Table 6: Bivariate analysis (correlation) between some variables and nutritional status (WFA, HFA, and WFH) of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed). WFA: weight for age; HFA: height for age; WFH: weight for height; r: correlation coefficient.
| Predictors | WFA | HFA | WFH |
| β (95.0% CI) | β (95.0% CI) | β (95.0% CI) |
| Sex of caregiver | -0.006 (-2.413, 2.09) | -0.051 (-3.175, 0.195) | 0.015 (-2.291, 3.464) |
| Age of caregiver | -0.043 (-0.766, 0.255) | 0.033 (-0.18, 0.584) | 0.9 (6.922, 8.309)** |
| Sex of child | -0.027 (-1.256, 0.602) | 0.027 (-0.371, 1.019) | -0.044 (-1.926, 0.47) |
| Age of child | 0.03 (5.76, 7.346)* | 0.927 (6.197, 7.024)** | -0.011 (-0.817, 0.605) |
| Marital status of the caregiver | -0.023 (-3.596, 1.966) | 0.019 (-1.391, 2.771) | 0.003 (-3.409, 3.698) |
| Educational status of the caregiver | 0.013 (-0.375, 0.507) | 0.02 (-0.221, 0.439) | -0.047 (-0.907, 0.227) |
| Socio-economic status of caregiver | 0.018 (-0.716, 1.098) | 0.017 (-0.496, 0.862) | 0.083 (0.053,2.407) |
| Occupation of caregiver | 0.005 (-1.218, 1.366) | 0.001 (-0.947, 0.987) | 0.001 (-1.622, 1.678) |
| Average monthly earnings | 0.074 (-0.074, 1.701) | -0.01 (-0.773, 0.555) | -0.006 (-1.217, 1.05) |
| Number of people | 0.922 (4.971, 5.963)** | 0.016 (-0.275, 0.467) | -0.001 (-0.656, 0.636) |
| Dietary diversity | 0.015 (-0.716, 1.038) | 0.023 (-0.395, 0.917) | -0.002 (-1.156, 1.109) |
| F | 50.355 | 103.201 | 60.473 |
| R | 0.901 | 0.948 | 0.916 |
| P-value | 0.000001 | 0.000001 | 0.000001 |
Table 7: Multivariate linear regression association between some variables and nutritional status (WFA, HFA, and WFH) of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed). WFA: weight for age; HFA: height for age; WFH: weight for height; β: beta (standard coefficient); CI: confidence interval.
| Variables | Frequency | Percentage (%) |
| Exclusive breastfeeding | Yes | 13 | 16.2 |
| No | 112 | 83.8 |
| Breastfed | | 85 | 79.8 |
| Non-breastfed | | 40 | 20.2 |
| Early initiation of breastfeeding | Yes | 25 | 18.0 |
| No | 100 | 82.0 |
| Breastfeeding regime (N = 121) | On-demand | 78 | 70.6 |
| Schedule time | 4 | 3.3 |
| When convenient | 43 | 26.1 |
| Was this child breastfed yesterday during the day or at night? | Yes | 72 | 60.7 |
| No | 53 | 39.3 |
| Was this child given any vitamin drops or other medicines as drops yesterday during the day or at night? | Yes | 66 | 52.9 |
| No | 59 | 47.1 |
Table 8: Infant and young child feeding practices among the caregivers of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
| Infant and Young Child Feeding practices | Yes | No | I don’t know |
| n (%) | n (%) | n (%) |
| Was this child given any vitamin drops or other medicines as drops yesterday during the day or at night? | 85 (65.9) | 40 (34.1) | 0 (.0) |
| Was this child giving water yesterday during the day or at night? | 100 (79.8) | 25 (20.2) | 0 (.0) |
| Was this child giving infant formula such as Cerelac, Friso Gold, or Aptamil NAN 1? | 44 (36.9) | 80 (62.5) | 1 (0.6) |
| Juice or juice drinks? | 45 (38.3) | 79 (61.1) | 1 (0.6) |
| Was this child giving yoghurt? | 61 (49.4) | 63 (50.0) | 1 (0.6) |
| Has this child eaten solid foods yesterday during the day or at night? | 75 (56.0) | 50 (44.0) | 0 (0.0) |
| Have you given this child pumpkin, carrots, squash, or sweet potatoes that are yellow or orange inside? | 86 (54.8) | 39 (45.2) | 0 (0.0) |
| White potatoes, white yams, cassava, or any other foods made from roots | 59 (48.2) | 64 (50.6) | 2 (1.2) |
| Any dark green leafy vegetables | 38 (45.0) | 87 (55.0) | 0 (.0) |
| Liver, kidney, heart, or other organ meats | 81 (63.1) | 44 (36.9) | 0 (.0) |
| Eggs, fresh or dried fish, shellfish, or seafood | 83 (66.8) | 40 (32.0) | 2 (1.2) |
| Any sugary foods such as chocolates, sweets, candies, pastries, cakes, or biscuits | 47 (40.5) | 78 (59.5) | 0 (.0) |
| Foods made with red palm oil, red palm nut, or red palm nut pulp sauce | 89 (69.0) | 35(30.4) | 1 (.6) |
| Did this child eat any solid, semi-solid, or soft foods yesterday during the day or at night? | 79 (61.1) | 46(38.9) | 0 (.0) |
| Did this child drink anything from a bottle with a nipple yesterday during the day or night? | 88 (68.9) | 37(31.1) | 0 (.0) |
Table 9: Complementary feeding practices among the caregivers of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano.
| Infant and young child feeding practices | WFA | HFA | WFH |
| R | p-value | R | p-value | R | p-value |
| Has this child ever been breastfed? | 0.051 | 0.513 | 0.000 | 1.000 | -0.002 | 0.982 |
| Was this child breastfed yesterday? | -0.121 | 0.118 | 0.000 | 1.000 | 0.059 | 0.446 |
| Was this child given any vitamin drops or other medicines as drops yesterday? | -0.062 | 0.429 | 0.000 | 1.000 | 0.100 | 0.198 |
| Has this child drank water? | -0.025 | 0.753 | 0.000 | 1.000 | 0.010 | 0.900 |
| Infant formula? | -.186* | 0.016 | 0.128 | 0.100 | 0.077 | 0.318 |
| Juice or juice drinks? | -.170* | 0.028 | 0.128 | 0.102 | -0.007 | 0.924 |
| Yoghurt? | -.215** | 0.005 | 0.124 | 0.112 | 0.005 | 0.948 |
| Has this child eaten solid foods yesterday? | -.226** | 0.003 | 0.127 | 0.102 | -0.049 | 0.529 |
| Have you given this child pumpkin, carrots, squash, or sweet potatoes that are yellow or orange inside? | -.191* | 0.013 | -0.064 | 0.415 | 0.014 | 0.861 |
| White potatoes, white yams, cassava, or any other foods made from roots | -0.140 | 0.071 | 0.058 | 0.456 | -0.049 | 0.532 |
| Any dark green leafy vegetables | -.175* | 0.023 | -0.064 | 0.415 | 0.031 | 0.692 |
| Liver, kidney, heart, or other organ meats | -.364** | 0.000 | -0.066 | 0.400 | 0.080 | 0.298 |
| Eggs, fresh or dried fish, shellfish, or seafood | -.216** | 0.005 | 0.000 | 1.000 | 0.043 | 0.581 |
| Any sugary foods such as chocolates, sweets, candies, pastries, cakes, or biscuits | -.153* | 0.047 | 0.000 | 1.000 | 0.030 | 0.696 |
| Foods made with red palm oil, red palm nut, or red palm nut pulp sauce | -0.026 | 0.735 | -0.066 | 0.397 | 0.018 | 0.819 |
| Did this child eat any solid, semi-solid, or soft foods yesterday? | -.214** | 0.005 | 0.130 | 0.096 | -0.022 | 0.775 |
| Did this child drink anything from a bottle with a nipple yesterday | -.334** | 0.000 | 0.000 | 1.000 | .218** | 0.005 |
Table 10: Bivariate analysis (correlation) between dietary practices and nutritional status (WFA, HFA, and WFH) of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed). WFA: weight for age; HFA: height for age; WFH: weight for height; r: correlation coefficient.
| Infant and young child feeding practices | WFA | HFA | WFH |
| Β (95.0% CI) | Β (95.0% CI) | Β (95.0% CI) |
| Has this child ever been breastfed? | 0.67 (0.581, 1.202)** | 0.08 (-0.101, 0.18) | -0.36 (-0.87,-0.094)* |
| Was this child breastfed yesterday? | -0.46 (-0.849, -0.305)** | -0.04 (-0.139, 0.107) | 0.14 (-0.158,0.521) |
| Was this child given any vitamin drops or other medicines? | 0.08 (-0.087, 0.28) | 0.09 (-0.047, 0.12) | 0.04 (-0.175,0.285) |
| Has this child drunk water? | 0.11 (-0.047, 0.353) | 0.02 (-0.082, 0.099) | -0.02 (-0.28,0.22) |
| Infant formula such? | -0.08 (-0.246, 0.078) | 0.13 (-0.023, 0.124) | 0.02 (-0.18,0.225) |
| Juice or juice drinks? | 0.13 (-0.067, 0.365) | 0.01 (-0.096, 0.102) | -0.06 (-0.338,0.202) |
| Yoghurt? | -0.05 (-0.268, 0.165) | 0.15 (-0.043, 0.155) | -0.05 (-0.321,0.22) |
| Has this child eaten solid foods yesterday? | -0.03 (-0.269, 0.2) | 0.22 (-0.021, 0.192) | -0.09 (-0.388,0.199) |
| Have you given this child pumpkin, carrots, squash, or sweet potatoes that are yellow or orange inside? | 0.22 (0.016, 0.471)* | -0.33 (-0.237, -0.027)* | -0.07 (-0.354,0.215) |
| White potatoes, white yams, cassava, or any other foods made from roots | -0.10 (-0.302, 0.094) | 0.13 (-0.042, 0.138) | 0.02 (-0.225,0.27) |
| Any dark green leafy vegetables | -0.10 (-0.286, 0.076) | -0.07 (-0.109, 0.055) | 0.04 (-0.183,0.269) |
| Liver, kidney, heart, or other organ meats | -0.42 (-0.665, -0.276)** | -0.10 (-0.132, 0.048) | 0.07 (-0.169,0.318) |
| Eggs, fresh or dried fish, shellfish, or seafood | -0.07 (-0.28, 0.115) | 0.06 (-0.068, 0.112) | 0.01 (-0.238,0.256) |
| Any sugary foods such as chocolates, sweets, candies, pastries, cakes, or biscuits | -0.11 (-0.303, 0.062) | -0.16 (-0.146, 0.022) | 0.13 (-0.091,0.365) |
| Foods made with red palm oil, red palm nut, or red palm nut pulp sauce | 0.28 (0.113, 0.553)** | -0.02 (-0.108, 0.092) | -0.08 (-0.365,0.185) |
| Did this child eat any solid, semi-solid, or soft foods yesterday? | -0.12 (-0.317, 0.046) | 0.16 (-0.017, 0.147) | -0.05 (-0.282,0.172) |
| Did this child drink anything from a bottle with a nipple yesterday? | -0.48 (-0.775, -0.356)** | -0.03 (-0.106, 0.084) | 0.36 (0.15,0.674)* |
| F | 7.102 | 1.021 | 1.222 |
| R | 0.68 | 0.33 | 0.359 |
| P-value | 0.0000001 | 0.44 | 0.255 |
Table 11: Multivariate linear regression association between dietary practices and nutritional status (WFA, HFA, and WFH) of children (0–23 months) accessing care in Hasiya Bayero Pediatric Hospital Kano. **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed). WFA: weight for age; HFA: height for age; WFH: weight for height; β: beta (standard coefficient); CI: confidence interval.
Discussion
This research work was carried out among 125 caregivers accessing care in Hasiya Bayero Pediatric Hospital Kano. As a whole, the participants were young, married, and uneducated as only 7.8% attended tertiary education. This is not in agreement with a study carried out in Burkina Faso [52] and Ethiopia [53]. The findings on marital status are in agreement with a study conducted by Mutua et al. [54] in Kenya. Most of the mothers were from low-income families, earning between 5,000–10,000 Naira as monthly income which is similar to a study conducted by Abdullahi et al. [55] and depend on business and farming for provision of food and other necessities. Other studies conducted in Nairobi have similar findings [54, 56, 57].
The minimum dietary diversity (MDD) indicator is the proportion of children (6–23 months of age) who receive foods from 4 or more food groups from a total of 7 food groups, namely, dairy products, legumes and nuts, flesh foods, eggs, vitamin A rich fruits and vegetables, cereals, reveal whether the child is receiving a complete and balanced diet or not. MDD was observed in 10.1% of the children between 6 and 23 months [58]. This is not similar to the present study as 39% of the children met MDD which is above the previous study.
The minimum meal frequency (MMF) indicator is the proportion of breastfed and non-breastfed children aged 6–23 months who receive solid, semi-solid, or soft foods (but also including milk feeds for non-breastfed children) the minimum number of times or more [58]. For breastfed children, the minimum number of times varies with age (two times if 6–8 months and three times if 9–23 months). For non-breastfed children, the minimum number of times does not vary by age (four times for all children aged 6–23 months). MMF was observed in about one-half (24.5%) of children aged 6–23 months [55]. This is different from the present study as cereals, grains, and their products were the most 71 (56.5%) consumed foods by the children, followed by legumes, nuts, and seeds which were consumed by 42 (30.0%) 7 or more per week while legumes (50%) and cereals (39%) were the most consumed food 4–6 times per week which are the minimum number of times to meet WHO recommendation for MMF [58].
The nutritional status of the children was poor. Up to 51.2%, 88.3%, and 65.1% of the infants were wasted, underweight, and stunted, respectively. This indicates that malnutrition started early in life with inappropriate IYCF practices such as the lack of exclusive breastfeeding and the introduction of complementary foods earlier than recommended as was discovered in this study. These complementary feedings are often inappropriate and harbor pathogenic microorganisms resulting in diarrhea and respiratory infections which could lead to malnutrition. A Cameroonian study noted that the level of underweight, stunting, and wasting among infants was high and attributed this to poor complementary feeding practices similar to our study [59]. Despite the low prevalence of exclusive breastfeeding (22.3% among infants aged 3 months and 0% among infants aged 4–6 months) reported from Morogoro Municipality in Tanzania, over 80% of the infants had normal weights, 13% were stunted, and 8% wasted; these were much lower than that observed in this study [60]. Our study shows that malnutrition, particularly wasting, underweight, and stunting was high. Weight for age (WLZ, WAZ, and LAZ) was significantly related to dietary diversity at p = 0.002, p = 0.001, and p = 0.019, respectively. This is in contrast to the results found at the national level during the 2015 nutrition survey [54] and is above the critical thresholds according to the WHO classification for assessing the severity of malnutrition by prevalence ranges among children under five years of age.
This research work shows that the educational status of the caregiver, socio-economic status of the caregiver, average monthly earnings of caregivers, and the number of people in the household of the caregivers were the only socio-demographic characteristics that were significantly associated with MDD. A significant portion of children from mothers who were technically educated (100%) were more likely (p-value = 0.049) to diversify their diet than children from mothers who had post-secondary education (0%), secondary (60.5%), and primary education (27.9%). Also, a significant portion of children from high (100%) socio-economic status was more likely (chi-square = 9.408, p-value = 0.009) to diversify their diet than the middle (12.9%) and lower (8.7%). The proportion of the study population who earns from 50,000–100,000 may also be likely to diversify their diet compared to those who earn below 10,000 (p = 0.001) and households that have a few number of people 1–5 people would likely diversify their foods than those that have a large number of people 16–20 in the household (p = 0.024). In contrast, some studies have shown that the level of the mother’s education should be sufficiently high (at least 9–13 years of schooling) to positively and statistically influence the nutritional status of the child [61, 62].
In our study for bivariate analysis between some variables and nutritional status (WFA, LFA, and WFL), there is evidence of the statistical relationship between the sex of caregivers and LFA at p = 0.041 and age of the caregivers showing the association between WFA, LFA, and WFL at p = 0.000, p = 0.044, and p = 0.000, respectively. The sex of children does not show a statistical difference between nutritional status while a significant association was observed between the age of children and HFA, p = 0.000. The study also shows a positive relationship between the mother’s education level and WFA and WFL at p = 0.006 and p = 0.033 while the number of people in the household also displayed statistical association between WFA and WFL with p-values of p = 0.000 and p = 0.000, respectively but no association was observed between dietary diversity and nutritional status of the children. This is in variance with the association described by Olusanya et al. [63]. The children whose caregivers have a large number of people in the household may be three times more likely to be malnourished. Thus, the fact that the caregiver with a large number of people in the household could result in a larger size of the household and less access to food and care for children compared to the caregivers that have less number of people in the household, which would expose them to greater vulnerability to malnutrition.
This present study found a statistical association between the age of the caregivers and WFL 0.9 (6.922, 8.309) at p = 0.01. Sex of children also does not show statistical differences between nutritional status in multivariate linear regression while significant association was observed between the age of children and WFA 0.03 (5.76, 7.346) and LFA 0.927 (6.197, 7.024) at p = 0.05 and p = 0.01, respectively. The number of people in the household also displayed a statistical association between WFA 0.922 (4.971, 5.963) with a p-value of p = 0.01 but no association was observed between dietary diversity. The findings in our study are different from several other studies [63, 64].
In this study, exclusive breastfeeding was practiced by only 16.2% of mothers out of the 125 participants. The majority (79.8%) of these children were still breastfeeding at the time of study but out of these children, only a few, about 18% were initiated to the breast within one hour of birth (early initiation of breastfeeding) as recommended by WHO and UNICEF that breastfeeding should be initiated within the first hour of birth [51]. Whereas the majority (70.6%) responded to breastfeeding on demand and a small proportion of 26.1% and 3.3% responded to breastfeeding “when convenient” and “breastfeeding at a scheduled time” respectively. Early initiation of breastfeeding was important, as colostrum is nutritive and essential, and “on‑demand breastfeeding” often led to earlier maximum milk production than feeding on a fixed schedule. However, a few of the mothers did not see colostrum as beneficial or important to the child’s well‑being; some even saw it as being harmful and resorted to expressing the milk and discarding it. Exclusive breastfeeding has been described as the single most important cost‑effective intervention to reduce infant morbidity and mortality, particularly in developing countries [65, 66].
Despite the strong evidence and wide publicity on the benefits of exclusive breastfeeding, the practice was low. This may be attributable to the fact that the majority of the mothers feared that babies would be thirsty because they believed that the water in the breast milk was not sufficient to avoid dehydration, especially during hot weather. The prevalence of breastfeeding practices noted in this study is similar to the findings in Papua New Guinea [67] but lower than the findings from the 2013 Nigerian Demographic and Health Survey [68] and other Nigerian studies from Kano [69], Birnin Kebbi [70], Enugu [62], and other developing countries such as Tanzania [71] and Iraq [72]. These findings showed 57% of children aged 6–9 months in India were exclusively breastfed up to 6 months, but lower than the findings of 74% for Bangladesh and 75% for Indonesia and Nigeria [72, 73]. The National Family Health Survey-4 (NFHS-4) also reported similar findings for India, which is 55% [74] which is in contrast with our present study (16.8%). Exclusively breastfed infants are at a lower risk of diseases like diarrhea, respiratory diseases, and pneumonia [75].
Inappropriate complementary feeding practices of the caregivers observed in our study were high as the majority of the mothers (83.8%) introduced complementary foods to their infants before 6 months of age. The early introduction of complementary feeds observed in this study may be attributable to the strong and widely held belief that breast milk is insufficient and hence the need to commence complementary feeding. The early introduction of complementary feeding observed in this study is higher than that reported in a similar study in Kano [69], Sokoto [76], and Southern Ethiopia [77]. According to this study’s findings, 83.8% of participants agreed that semi-solid foods should be introduced at 6 months and that breastfeeding should not be stopped when these foods are consumed. Similar to this, Assefa et al. [78] indicated that mothers were aware that complementary meals should not be offered to children until they were six months old [79].
However, their level of knowledge may impact how caregivers feed their children, hence it is recommended to strengthen IYCF education to a wider range of the public, including families and communities. WHO recommends that infants should start receiving complementary foods at 6 months of age in addition to breast milk. Furthermore, in the present study, only 83.8% of children aged above 6 months received complementary feeding, which is in contrast to a previous study [66]. The NFHS-4 also reported only 43% of children aged 6–8 months receiving complementary foods [80].
In this study, we observed a statistical relationship between IYCF practice indicators and WFA at p = 0.01 and p = 0.05. This could be due to the effect of “unobserved factors” that may influence both the mother’s education and the child’s nutritional status. Only bottle-feeding practices showed an association with WFL at p = 0.01. There was no statistical association between nutritional status and child ever breastfed, child breastfed yesterday, and administering of vitamin drops and medicine at p-values of p = 0.982, p = 0.446, and p = 0.198, respectively. This is not similar to a study carried out by Ruel et al. [80]. This result suggests improving feeding practices in this age group in our context. Our results are similar to those of Saaka et al. [81], which have shown a strong association between the IYCF practices and the Z score height/age in children aged 6–12 months.
Sawadogo et al. [82] have also documented similar results by finding associations between the IYCF index and Z score weight/height in children aged 6–11 months and with Z score height/age in 6–23 months. By cons, Zhang et al. [83] in a study in a rural China community among children aged 6–11 months observed no association between the Z Score height/age and the IYCF index but he noted, however, an association between the Z Score weight/age and the IYCF practices. Multivariate linear regression association between dietary diversity and nutritional status (WFA, LFA, and WFL), this present study found a statistical association between child ever breastfed and WFA 0.67 (0.581, 1.202) and WFL -0.36 (-0.87, -0.094) at p = 0.01, p = 0.05 [84]. A statistical difference was observed between child breastfed yesterday and WFA -0.46 (-0.849, -0.305) while a significant association was also observed between pumpkin, carrots, squash, or sweet potatoes and WFA 0.22 (0.016, 0.471) and LFA -0.33 (-0.042, -0.027) at p = 0.05 and p = 0.01, respectively. While the liver, kidney, heart, or other organ meats and vegetables oil also displayed statistical association with WFA -0.42 (-0665, -0.276) and 0.28 (0.113, 0.553) with p-values of p = 0.01, p = 0.05, there is a significant difference between bottle feeding practices and WFA -0.48 (-0.775, -0.356) and WFL 0.36 (0.15, 0.674) but no association was observed between solid, semi-solid and soft foods and nutritional status of the children. The finding of the multivariate analysis of the nutritional status of our study was at variance with the findings described by Bharati et al. [85]. The higher rate of malnutrition observed in this study could be due to inappropriate feeding practices.
Conclusion
The nutritional status of children (0–23 months) registered for care in Hasiya Bayero Pediatric Hospital was below the WHO cut-off. Initiation of breastfeeding at birth (18%), exclusive breastfeeding (16.2%), and complementary feeding practices among the caregivers of the children (0–23 months) fell below the WHO recommendation. This study revealed poor IYCF practices in Hasiya Bayero Pediatric Hospital Kano. Therefore, more attention needs to be paid to the specific behaviors surrounding feeding practices and other constraints to children accessing care in Hasiya Bayero Pediatric Hospital Kano.
References
- Nigeria Governers Forum. National demographics for health survey. 2019.
- National Nutrition and Health Survey. 2018.
- Definition of dietary intake. World Health Organization; 2017; 237 pages. (WHO/NUT/98.1).
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3-9.
- Moeller SM, Reedy J, Millen AE, et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. J Am Diet Assoc. 2007;107(7):1233-239.
- Smithers LG, Brazionis L, Golley RK, et al. Associations between dietary patterns at 6 and 15 months of age and sociodemographic factors. Eur J Clin Nutr. 2012;66(6):658-66.
- Drewnowski A. Taste preferences and food intake. Annu Rev Nutr. 1997;17:237-53.
- Abdullahi H, Olamuyiwa A O, Ndidi US, et al. Infant and young-child feeding practices for under-two children involved in community infant and young child feeding programme in Zaria, Nigeria. FUDMA Journal of Sciences (FJS). 2022;6(1):27-32.
- Bowman B, Russel R. Present Knowledge in Nutrition. International life science institute Press. 2007;99(1):214.
- WHO, UNICEF. Indicators for assessing infant and young child feeding practices: definitions and measurement methods. 2021.
- UNICEF, . Informal settlements are made up of improvised dwellings often made from scrap materials. (United Nation Children fund). 2010.
- Liu J, Ai YX, Hanlon A, et al. Micronutrients deficiency and associated sociodemographic factors in Chinese children. World J Pediatr. 2011;7(3):217-23.
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969-987.
- The state of the world’s children. 2019.
- The Community Infant and Young Child Feeding Counselling Package. 2017.
- Bhutta ZA, Ahmed T, Black RE, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417-40.
- Saha KK, Frongillo EA, Alam DS, et al. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. 2008;87(6):1852-859.
- Jones G, Steketee RW, Black RE, et al. How many child deaths can we prevent this year?. Lancet. 2003;362(9377):65-71.
- Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. 1995.
- Hop LT, Gross R, Giay T, et al. Premature complementary feeding is associated with poorer growth of vietnamese children. J Nutr. 2000;130(11):2683-690.
- Global strategy for infant and young child feeding. 2003.
- Ministry of Health and Family Welfare. Integrated management of neonatal and childhood illness. 2003.
- Sinhababu A, Mukhopadhyay DK, Panja TK, et al. Infant- and young child-feeding practices in Bankura district, West Bengal, India. J Health Popul Nutr. 2010;28(3):394-09.
- Global strategy for infant and young child feeding. 2003.
- Mendelson S, and Chaudhuri S. Child Malnutrition in India. 2009.
- Islamic Finance Nigeria. IFN Nigeria Report2020. 2020.
- Svedberg P. Why malnutrition in shining India persists. 2008.
- Kano State. 2020.
- National Results Population By State And Sex. 2011.
- “Kano’s 500-year-old market” 2021.
- Nigeria Embassy. Embassy of the Federal Republic of Nigeria History.
- When did it Gwandara split from Hausa?.
- History Today. The Fall of Kano.
- Research Gate. Kano Metropolis.
- Kano State. Kano Markets. 2020.
- Hausa Language Variation and Dialects.
- Adoti, and Olive. “10 Top languages spoken in Nigeria (plus the states)”. Legit.ng – Nigeria news. Archived from the original on 23 April 2021. Retrieved; 2021.
- Nossiter and Adam. Bombs Strike Bus Station in Nigeria. 2021.
- BBC News. Nigeria suicide bombers target Potiskum and Kano buses. 2015.
- Kano mosque attack kills dozens. BBC News. 2014.
- Obasanjo Assesses Riot Damage in Kano – 2001-10-16. Voice of America News. 2009.
- Kano: Nigeria’s ancient city-state. BBC online. 2004.
- Nigerian singer sentenced to death for blasphemy in Kano state. BBC News. 2020.
- Sanusi MM, Derrick DM. Geographies of poverty in Kano State: The role of GIS in identifying and mapping multidimensionally deprived households. Research Gate. 2015.
- Kano mosque attack kills dozens. 2014.
- Sanusi MM, Derrick DM. Geographies of poverty in Kano State: The role of GIS in identifying and mapping multidimensionally deprived households. ResearchGate. 2015.
- In Preparation for Elimination of Mother-to-Child Transmission of HIV. State-Wide Rapid Health Facility Assessment. 2013.
- Universities and colleges in Kano State.
- Junaidu D. A history of the transformation of water supply in Kano, Nigeria, 1924-1960. Journal of Advances in Humanities and Social Sciences. 2019;5(1):12-20.
- Umar AB, Mohammed NY. Hospital Waste Management Practices: A Case Study of Primary HealthCare Centers, In Fagge Local Government Area, Kano State. IOSR Journal of Nursing and Health Science (IOSR-JNHS). 2014;3(6):26-33.
- Global Strategy on Infant and Young Child Feeding. 2003.
- Pollitt E, Gorman KS, Engle PL, et al. Nutrition in early life and the fulfillment of intellectual potential. J Nutr. 1995;125(4):1111-118.
- Infant and Young Child Feeding. 2022.
- Mutua MK, Kimani-Murage E, Ettarh RR. Childhood vaccination in informal urban settlements in Nairobi, Kenya: who gets vaccinated? BMC Public Health. 2011;11(1):6.
- Abdullahi H, Olamuyiwa AO, Ndidi US, et al. INFANT AND YOUNG-CHILD FEEDING PRACTICES FOR UNDER-TWO CHILDREN INVOLVED IN COMMUNITY INFANT AND YOUNG CHILD FEEDING PROGRAMME IN ZARIA, NIGERIA. FUDMA Journal of Sciences. 2022;6(1):27-32.
- Ochola SA. Evaluation of two counseling strategies promoting exclusive breastfeeding among HIV-negative mothers in Kibera slum, Nairobi, Kenya: A randomized controlled trial. PhD Thesis. Stellenbosch University, South Africa.
- Adere JW. Feeding practices and nutritional status of Children 6-36 months in muslim and christian Households: a human rights perspective (a case study of Kibera in Nairobi, Kenya). University of Nairobi. 2006.
- Indicators for assessing infant and young child feeding practices: part 1 definition. 2008.
- Mananga MJ, Kana-Sop MM, Nolla NP, et al. Feeding Practices, Food and Nutrition Insecurity of infants and their Mothers in Bangang Rural Community, Cameroon. Journal of Nutrition and Food Sciences. 2014;4(2)-7.
- Safari JG, Kimambo SC, Lwelamira JE. Feeding practices and nutritional status of infants in Morogoro Municipality, Tanzania. Tanzan J Health Res. 2013;15(3):178-75.
- Umar AS, Oche MO. Breastfeeding and Weaning Practices in an Urban Slum, North Western Nigeria. International Journal of Tropical Disease and Health. 2013;3(2):114-125.
- Anoshirike C O, Ejeogo C P, Nwosu O I, et al. Infant Feeding Practices Among Mothers and Their Infants Attending Maternal And Child Health In Enugu, Nigeria. Journal Biology Agriculture Healthcare. 2014;4(10): 130‑9.
- Olusanya BO, Wirz SL, Renner JK. Prevalence, pattern and risk factors for undernutrition in early infancy using the WHO Multicentre Growth Reference: a community-based study. Paediatr Perinat Epidemiol. 2010;24(6):572-83.
- Akombi BJ, Agho KE, Hall JJ, et al. Stunting and severe stunting among children under-5 years in Nigeria: A multilevel analysis. BMC Pediatr. 2017;17(1):15.
- Chirande L, Charwe D, Mbwana H, et al. Determinants of stunting and severe stunting among under-fives in Tanzania: evidence from the 2010 cross-sectional household survey. BMC Pediatr. 2015;15(165).
- Global Strategy for Infant and Young Child Feeding. 2003.
- Kuzma J. Knowledge, attitude and practice related to infant feeding among women in rural Papua New Guinea: a descriptive, mixed method study. International Breastfeeding Journal. 2013;8:16.
- Indicators for assessing infant and young child feeding practices: definitions and measurement methods. 2021.
- Sholeye OO, Akintunde A, Elijah B, et al. Knowledge of infant feeding among mothers in sagamu, southwestern Nigeria: implications for nutrition education. AMERICAN JOURNAL OF FOOD AND NUTRITION. 2016;6:69-76.
- Lee AC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):26-36.
- O’Leary M, Edmond K, Floyd S, et al. A cohort study of low birth weight and health outcomes in the first year of life, Ghana. Bull World Health Organ. 2017;95(8):574-83.
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6):588-95.
- Progress for Children: Achieving the Millenniums Development Goals with Equity. 2010.
- Ministry of Health and Welfare. International Institute for Population Sciences & Infant and Child Feeding. 2017.
- Infant and Young Child Feeding. 2022.
- Maïga E. The Impact of Mother’s Education on Child Health and Nutrition in Developing Countries: Evidence from a Natural Experiment in Burkina Faso. African Center for Economic Transformation. 2011. Report No.: I15, I25, I28.
- Makoka D. The Impact of Maternal Education on Child Nutrition: Evidence from Malawi, Tanzania, and Zimbabwe. DHS working papers. 2013;84.
- Assefa DG, Woldesenbet TT, Molla W, et al. Assessment of knowledge, attitude and practice of mothers/caregivers on infant and young child feeding in Assosa Woreda, Assosa Zone, Benshangul Gumuz Region, Western Ethiopia: a cross-sectional study. Arch Public Health. 2021;79(1):170.
- Shrestha S, Pokhrel M, Mathema S. Knowledge. Knowledge, Attitude and Practices among Mothers of Children 6 to 24 months of Age Regarding Complementary Feeding. JNMA J Nepal Med Assoc. 2020;58(230):758-63.
- Ruel MT, Menon P. Child feeding practices are associated with child nutritional status in Latin America: innovative uses of the demographic and health surveys. J Nutr. 2002;132(6):1180-187.
- Saaka M, Wemakor A, Abizari AR, et al. How well do WHO complementary feeding indicators relate to nutritional status of children aged 6-23 months in rural Northern Ghana?. BMC Public Health. 2015;1157.
- Sawadogo PS, Martin-Prével Y, Savy M, et al. An infant and child feeding index is associated with the nutritional status of 6- to 23-month-old children in rural Burkina Faso. J Nutr. 2006;136(3):656-63.
- Zhang J, Shi L, Wang J, et al. An infant and child feeding index is associated with child nutritional status in rural China. Early Hum Dev. 2009;85(4):247-52.
- Ogbo FA, Agho KE, Page A. Determinants of suboptimal breastfeeding practices in Nigeria: evidence from the 2008 demographic and health survey. BMC Public Health. 2015;15:259.
- Bharati S, Pal M, Chakrabarty S, et al. Trends in socioeconomic and nutritional status of children younger than 6 years in India. Asia Pack. Asia Pac J Public Health. 2011;23(3):324-40.

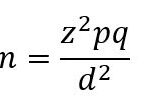
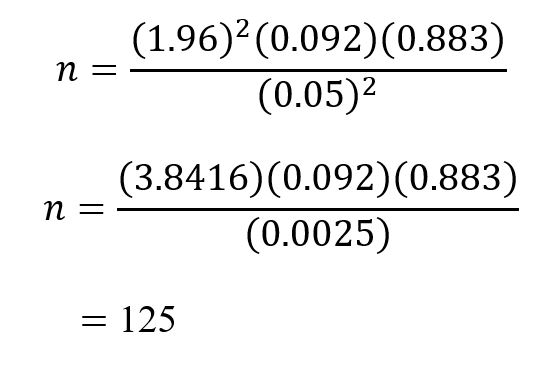 Sampling technique
Sampling technique