diffuse large B-cell lymphoma, tumor, Wunderlich syndrome, flank pain
DLBCL: diffuse large B-cell lymphoma, ABC: activated B-cell-like, GCB: germinal center B-cell-like, PRL: primary renal lymphoma, SEER: Surveillance, Epidemiology, and End Results, MALT: mucosa-associated lymphoid tissue, CT: computed tomography, ICU: intensive care unit, CNS: central nervous system
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma worldwide representing 30–40% of all cases. Patients often present with a rapidly growing tumor mass in single or multiple sites [1], it is also the most common type of hematologic malignancy and is characterized by a striking degree of genetic and clinical heterogeneity [2]. Gene expression profiling defined the activated B-cell-like (ABC) and germinal center B-cell-like (GCB) subgroups of DLBCL leaving up to 20% of cases unclassified [3]. This phenotypic distinction is associated with overall survival after standard therapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy [3]. Primary renal lymphoma (PRL) is a very rare disease that comprises less than 1% of extranodal lymphomas, with only a few cases reported in the literature [4]. Although renal involvement has been reported in up to 60% of patients with non-Hodgkin lymphoma [5], PRL is unusual and is often mistaken for renal cell carcinoma [5]. Using the Surveillance, Epidemiology, and End Results (SEER) database, Chen et al. [4] identified 723 patients in a 33-year period, with an incidence of 0.053/100,000 person-years. The most common histological subtype of PRL is DLBCL [4] but lymphoma of mucosa-associated lymphoid tissue (MALT) of the kidney [6], intravascular large B-cell lymphoma of the kidney [7], and bilateral renal involvement have been reported [8]. As a normal kidney lacks lymphoid tissue [4] it has been postulated that PRL originates from the renal capsule (rich in lymphatic tissue) and penetrates renal parenchyma [4]. Clinical presentation is non-specific and includes gross hematuria, flank pain, weight loss, fever, and/or kidney failure [4]. Wunderlich syndrome is a rare clinical syndrome of spontaneous nontraumatic renal subcapsular and retroperitoneal hemorrhage [9]. Classical presentation often includes sudden onset of flank pain that can be confused with a renal colic, palpable flank mass, and hypovolemic shock, a series of symptoms known as Lenk’s triad [9]. Neoplasms are the most common underlying pathology in up to 60% of cases and include renal angiomyolipoma and renal cell carcinoma [9]. Other etiologies include rupture of the renal artery or an arteriovenous malformation, polyarteritis nodosa, cystic medial necrosis, segmental arterial mediolysis, and cystic rupture [9]. Carl Wunderlich first described a patient with spontaneous subcapsular and perinephric bleeding without preceding trauma in 1856 [10]. Initial treatment for Wunderlich syndrome includes a conservative non-surgical approach, selective arterial embolization, and laparoscopic or open surgical intervention [11–13].
A 51-year-old male patient presented to the Emergency Department complaining of a sudden, severe left flank pain that started 60 minutes prior with nausea and vomiting, the pain radiated to the back and was sharp and constant; he denied having gross hematuria. On physical examination he had tachycardia with a heart rate of 115 beats per minute, with an arterial blood pressure of 90/60 mmHg; a left abdominal palpable mass was identified with tenderness but with no signs of peritoneal irritation and had a positive left Giordano sign. At arrival, he had a hemoglobin of 14 g/dL, leukocytes 12,200, platelets 286,000, LDH 532, and normal kidney function. An abdominal computed tomography (CT) with IV contrast revealed a large retroperitoneal mass arising from the inferior half of the left kidney measuring 18 × 17 × 12 cm, displacing the aorta to the right, and the intraperitoneal contents anteriorly due to the large tumor volume (Figure 1). The mass showed a heterogeneous pattern with hematic components suggesting the presence of active or recent bleeding; the majority of the tumoral component is isodense, with poor or no enhancement with the IV contrast. The liver, spleen, and contralateral kidney are reported normal. It was decided to perform an open procedure due to the hemodynamic instability and the tumor’s volume through a left subcostal hemi-chevron incision, identifying a large hematoma that infiltrated the descending mesocolon without compromising its vascularity or the integrity of the bowel; the Toldt’s fascia was incised and a large number of clots was extracted, with the anatomy completely lost, hardly being able to identify normal structures or the kidney properly. Vascular structures resembling renal, adrenal, and lumbar blood vessels were carefully dissected and controlled as well as the left ureter until the mass and clots were removed entirely. No active bleeding was identified after the nephrectomy; a closed system drainage was inserted and after the abdominal wall closure the patient was admitted stable but intubated and with mechanical ventilation to the intensive care unit (ICU). 24 hours later he was extubated and after four days was discharged from the ICU to general admissions and was discharged from the hospital 72 hours later.
The pathology report of the kidney tumor showed a DLBCL with markers CD 10 and 20 positive in the membrane of the malignant cells, MUM1 positive and Ki67 in 70% of neoplastic cells (Figure 2). The patient was referred to hematology for assessment and further treatment. A spinal tap ruled out the presence of malignant cells in the central nervous system (CNS). A large B-cell lymphoma was diagnosed with a high risk of CNS progression, so he started chemotherapy with an R-CHOP regimen (rituximab, cyclophosphamide, cardioxane, doxorubicin, and vincristine) every 21 days for 6 cycles. Each cycle included intrathecal methotrexate, cytarabine, and dexamethasone as CNS prophylaxis. The last cycle was administered on October 11th, 2021. The patient experienced a complete response documented by an F-18 FDG PET scan reported as a Deauville 1 score (no uptake), and the most recent F-18 FDG PET scan performed in November 2023 also reported a Deauville 1 score concluding that there is no evidence of disease (Figure 3). His renal function remains normal with the most recent laboratories showing a creatinine at 0.8 mg/dL, urea at 32 mg/dL, urea nitrogen at 15 mg/dL, and a BUN/creatinine ratio at 18, all normal parameters. He remains in clinical surveillance every 3 months with annual F-18 FDG PET scans.
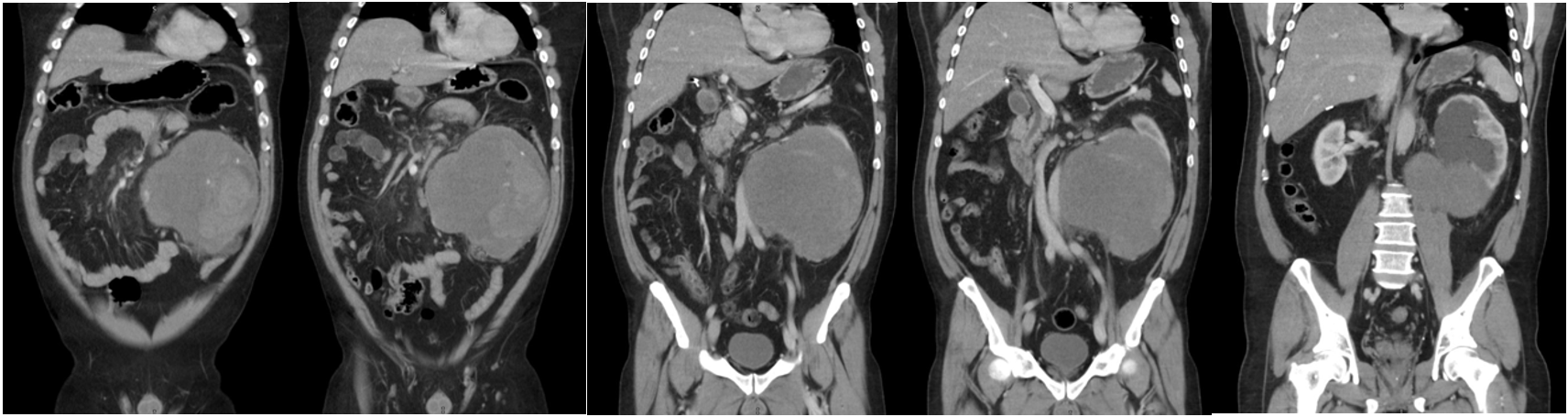 Figure 1: Large retroperitoneal mass arising from the inferior half of the left kidney displacing the aorta to the right and the intraperitoneal contents anteriorly.
Figure 1: Large retroperitoneal mass arising from the inferior half of the left kidney displacing the aorta to the right and the intraperitoneal contents anteriorly.
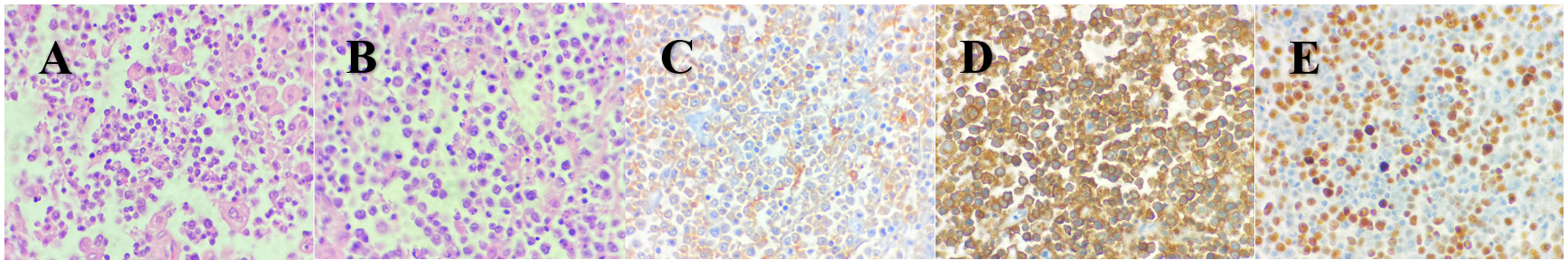 Figure 2: A and B) Poorly differentiated malignant neoplasia conformed by small cells disposed in a diffuse manner with a scarce, eosinophilic cytoplasm. C) CD10 positive. D) CD20 positive. E) Ki67 positive.
Figure 2: A and B) Poorly differentiated malignant neoplasia conformed by small cells disposed in a diffuse manner with a scarce, eosinophilic cytoplasm. C) CD10 positive. D) CD20 positive. E) Ki67 positive.
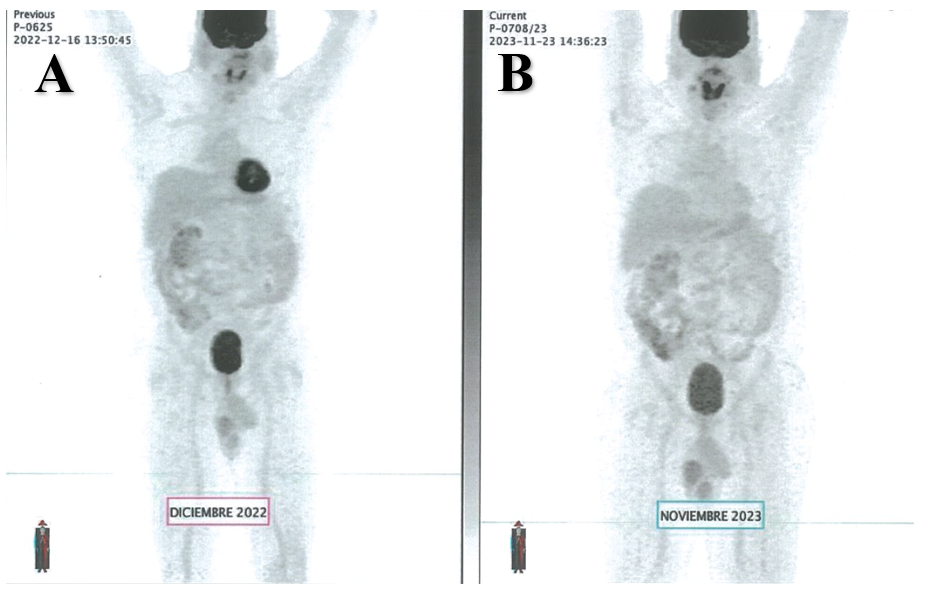 Figure 3: A) December 2022 F-18 FDG PET scan showing no uptake (Deauville 1). B) November 2023 F-18 FDG PET scan showing no uptake (Deauville 1).
Figure 3: A) December 2022 F-18 FDG PET scan showing no uptake (Deauville 1). B) November 2023 F-18 FDG PET scan showing no uptake (Deauville 1).



