Introduction: Tuberculosis (TB) in Mexico is considered an endemic public health problem. Infection in adults is caused 95% of the time by Mycobacterium tuberculosis (Mtb). Although non-invasive diagnostic techniques are the first choice, the diagnostic yield of these techniques does not always allow definitive diagnosis, so sometimes, surgical biopsy is necessary.
Objective: To describe two cases of pleural TB with a difficult diagnosis in which non-invasive diagnostic tests were inconclusive.
Conclusion: In the presence of pleural or pulmonary nodules or tumors, we should consider TB as a differential diagnosis, as well as in those patients whose clinical suspicion is high and non-invasive methods have not been diagnostic. Therefore, the surgical approach is still an option for these patients.
tuberculosis, pulmonary, pleural, diagnosis, biopsy
TB: tuberculosis, Mtb: Mycobacterium tuberculosis, PPV: positive predictive value, NPV: negative predictive value, AFB: acid-fast bacilli, ADA: adenosine deaminase, PCR: polymerase chain reaction, COVID-19: coronavirus disease 2019
Tuberculosis (TB) is an infectious contagious disease caused by Mycobacterium tuberculosis (Mtb), which can be acute, subacute, and chronic, the latter being the most frequent form.
Acute TB occurs mainly in immunocompromised patients and children with the following triad: fever, night sweats, and unintentional weight loss in 75%, 45%, and 55%, respectively. It can manifest in any organ of the body; however, the most common forms are miliary, meningeal, abdominal, and pulmonar [1].
On the other hand, chronic TB is characterized by the formation of granulomas, which are formed by macrophage-activating lymphocytes, epithelioid cells, and giant cells located concentrically to surround and try to phagocytose the bacilli. These granulomas can affect different organs (25% of patients can present extrapulmonary TB), with lung disease (pulmonary TB) predominating in 80–85% of them [2].
According to the 2022 global tuberculosis report, worldwide incidence increased by 4.5% for the first time in 20 years, as did mortality, from 1.5 million in 2020 to 1.6 million in 2021. In Mexico, pulmonary TB is considered an endemic public health problem; infection in adults is caused 95% of the time by Mtb and to a lesser extent by Mycobacterium bovis. The TB rate in the early years of the 21st century has ranged between 13 and 17 cases per 100,000 inhabitants. In some states, the rate is greater than 30 per 100,000 residents. In Mexico, the most common clinical forms are pulmonary, nodal, renal, and meningeal [2, 3].
Pleural affection can occur as a complication of pulmonary TB during reactivation but is usually a manifestation of the primary infection as a result of retrograde pleural invasion by the lymphatic route of mycobacteria in the underlying lung tissue or by pleural seeding during the hematogenous phase or a combination of both. The presence of mycobacteria in the pleural, usually in small quantities, triggers the inflammatory response typical of TB. This provokes the formation of exudative-type pleural effusion. This fluid frequently does not contain mycobacteria [3, 4]. The accumulation of fluid in the pleural space due to Mtb infection occurs as a result of a combination of multiple factors. The triggering factor is the rupture of a subpleural caseous focus, which causes the entry of Mtb into the pleural space [5]. The presence of caseating granulomas containing acid-fast bacilli (AFB) on histological examination of the pleural surface is diagnostic of tuberculous pleuritis.
Patients usually present with fever, productive cough, night sweats, and weight loss that accompany an increase in lymph node volume. In patients with few symptoms, the diagnosis of extrapulmonary TB should be suspected when there is predominantly lymphocytic pleural effusion with negative bacterial cultures and chronic cervical lymphadenopathy, among others [6].
For diagnosis, microbiological analysis of pleural fluid must be performed through thoracocentesis. The fluid should correspond to a mononuclear lymphocytic exudate with decreased glucose levels. The table (Table 1) describes the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic performance of the different tests [7–12]. It is important to highlight that the gold standard for diagnosis is biopsy with visualization of the Bacillus and the presence of caseating granulomas and that the low sensitivity and limited diagnostic capacity of Xpert are probably due to a low load of organisms in pleural fluid. A combined strategy of pleural fluid biochemistry and closed needle biopsy [histology with AFB stain and mycobacterial culture] was the next best diagnostic test for medical thoracoscopy, with a sensitivity and specificity of 93% and 100%, respectively [11, 12].
In the table below (Table 1), the different diagnostic methods are resumed.
| Method | Diagnostic performance | SE (%) | S (%) | PPV (%) | NPV (%) |
| Thoracocentesis |
| Pleural fluid culture | 7–10 | 10–35 | 100 | – | – |
| Pleural fluid cytology | – | 66 | 100 | – | – |
| ADA | – | 92–95 | 90 | – | – |
| QuantiFERON |
| QFT-G | – | 78–80 | 95 | – | – |
| QFT-GIT | – | 67–70 | 95 | – | – |
| QFT-Plus | – | 95 | 95 | – | – |
| Baciloscopy | – | 25–75 | 90–95 | – | – |
| PCR |
| NAAT | – | 28–81 | 90 | 100 | – |
| GeneXpert MTB/RR | – | 51,4–62 | 98,6 | 56,4 | 75,6 |
| IFN-γ in pleural fluid | 7–58 | 89 | 97 | – | – |
| Closed pleural biopsy (Abrams, Cope and Ramel Needle) |
| Blind biopsy | 50–80 | 93 | 100 | 98 | 77 |
| Image guided (ultrasound or CT) | 90–93,4 | 98–100 | – | – | – |
| Open pleural biopsy |
| Thoracoscopy | 93.8–100 | 93–100 | 100 | – | – |
| Thoracotomy | 93.8–100 | 93–100 | 100 | – | – |
| Other |
| Sputum culture | 7–30 | 48 | – | – | – |
| Pleural fluid culture + pleural fluid cytology | – | 79 | – | – | – |
| ADA + L:N | – | 89 | 100 | – | – |
| ADA + IFN-γ | – | – | 100 | 100 | – |
| Closed biopsy + pleural fluid culture | – | 85 | 100 | – | – |
| Closed biopsy + ADA | – | 93 | 100 | – | – |
| Closed biopsy + ADA + L:N | – | 93 | 100 | – | – |
| Closed biopsy + cytology + AFB staining | – | 100 | 100 | – | – |
Table 1: Diagnostic methods for pleural tuberculosis and their diagnostic performance. ADA: adenosine deaminase; AFB: acid-fast bacilli; CT: computed tomography; IFN-γ: interferon-gamma; L:N: lymphocyte/neutrophil ratio; MTB/RR: Mycobacterium tuberculosis/rifampicin resistance; NAAT: nucleic acid amplification test; NPV: negative predictive value; PPV: positive predictive value; PCR: polymerase chain reaction; QFT-G: QuantiFERON-TB Gold; QFT-GIT: QuantiFERON-TB Gold in Tube; QTF-Plus: QuantiFERON-TB Gold Plus; SE: sensibility; S: specificity. [5, 8–11, 13–20]
2.1 Case 1
A 46-year-old male with a history of a sister diagnosed with non-Hodgkin lymphoma, operating as a clinical laboratory worker for 4 years, newly diagnosed type 2 diabetes mellitus and severe pneumonia due to COVID-19 without requiring mechanical ventilation in December 2022. Starts current condition after COVID-19 infection with dyspnea, two months later with dry cough and unquantified fever predominantly in the evening, myalgia, arthralgia, and nausea; a simple chest tomography is performed where a right pleural effusion of approximately 40% was detected with passive atelectasis. Diagnostic and therapeutic thoracocentesis was performed, resulting in mononuclear exudate, adenosine deaminase (ADA) of 44.84 U/L, negative polymerase chain reaction (PCR) for TB, negative culture of bacteria and mycobacteria and inconclusive pathology result. Therefore, a surgical approach was proposed for biopsy. Due to the patient´s pleural characteristics, a thoracotomy was performed where parietal pleura, diaphragm, and pericardium with “breadcrumb” characteristics were visualized, as well as pleural effusion and complete lung entrapment with fixed adhesion. Decortication was performed with a parietal pleura and diaphragm biopsy sampling, subsequent closure of the intercostal space with sliding stitches, aponeurosis by two simple planes, and skin with subdermal stitching. Pathology reported: pleural and diaphragm with fibroadipose tissue with chronic granulomatous inflammation due to Mtb (presence of Bacillus in Ziehl-Neelsen stain). The patient was treated with a “Dotbal” (rifampicin, isoniazid, ethambutol, pyrazinamide) regimen, with an intensive phase of 4 tablets orally every 24 h for 60 days. The patient is currently asymptomatic, with radiographic improvement (Figure 1).

Figure 1: Clinical case 1.
2.2 Case 2
A 52-year-old female with a positive history of smoking with a smoking index of 12 packs per year, systemic arterial hypertension of 10 years duration, and cardiac arrhythmia (unspecified) of 5 years duration. The current condition began by going to the pneumologist to quit smoking, so a chest scan was performed, and a 3 cm right pleural nodule was detected. Given the smoking rate and characteristics of the lesion, its resection was suggested. It was electively scheduled for thoracotomy, where loose adhesions were visualized and removed, and a wedge-shaped lesion of approximately 3 × 3 × 2 cm was removed from the posterior segment of the right upper lobe and subsequent hermetic closure of lung tissue. By pathology, pulmonary TB was reported with chronic granulomatous inflammation, with foreign body type multinucleated right giant cells, anthracosis, and extensive coagulative necrosis; Ziehl-Neelsen staining was performed with a focally positive result. Treatment was started with a “Dotbal” regimen, with an intensive phase of 4 tablets orally every 24 h for 60 doses, currently asymptomatic (Figure 2).
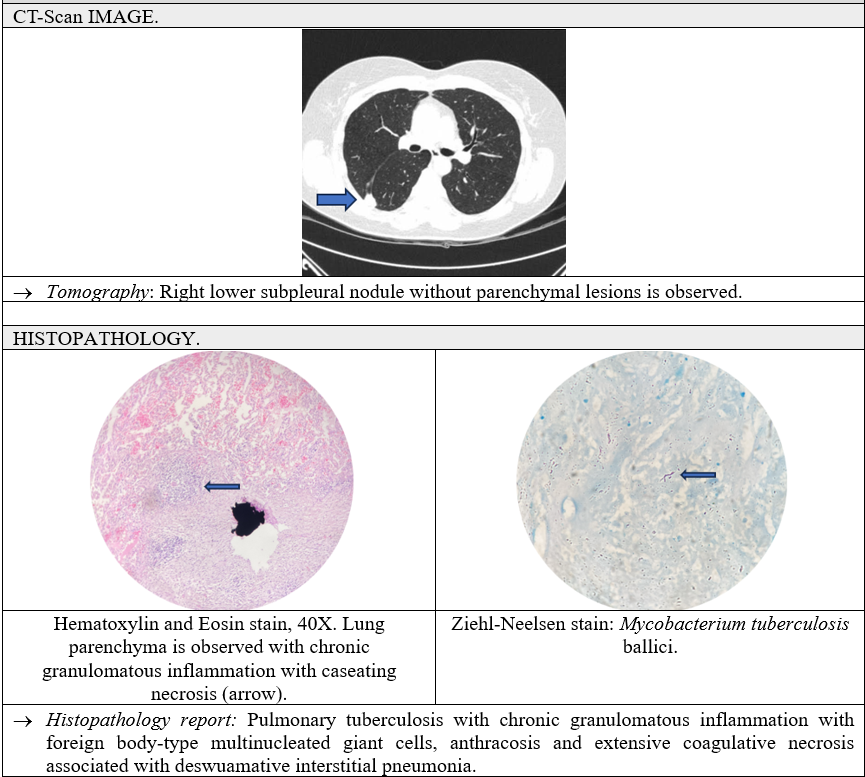
Figure 2: Clinical case 2.
TB is a preventable and curable disease, so it is necessary to strengthen the effectiveness of its timely detection and its subsequent treatment to reduce its incidence; with a mortality rate in Mexico of 2.0 per 100,000 inhabitants for the year 2019, according to the World Health Organization, currently, there are multiple non-invasive methods for the detection of this disease, among which are based on cellular, molecular, and serological components that show high sensitivity and specificity; however, the diagnostic test that has a sensitivity and specificity close to 100% is performing surgical lung/pleural biopsy, where it is possible to directly visualize the presence of the caseating Bacilli and granulomas. In this series of cases, two cases are presented, which represent a diagnostic challenge. In the case of the first patient, he had a family history of lymphoma and had worked in a bacteriological laboratory, he presented a mononuclear exudate-type pleural effusion with a borderline ADA for the Mexican population, negative PCR, and cultures with the differential diagnosis being TB and neoplasia. Due to the above, a pleural biopsy was performed, and during surgery, the parietal pleural was seen completely covered with granulomas, confirming the presence of the Bacillus by a histopathology study. In the second case, the patient had a high smoking rate and no symptoms suggestive of TB, so a tumor lesion was initially suspected, and a diagnostic approach focused on neoplastic etiology was conducted, obtaining negative tumor markers. A guided biopsy was considered; however, the lesion was in a site that was difficult to access, so the decision to perform surgery was made for the resection of the lesion, and the presence of the Bacilli was confirmed by histopathology.
Although non-invasive diagnostic techniques are the first choice for the diagnosis of TB, their diagnostic performance does not always allow a definitive diagnosis, which is why surgical biopsy is sometimes needed. This is why, in the presence of pleural or lung nodules or tumors, we must consider a TB differential diagnosis, as well as in those patients whose clinical suspicion is high and non-invasive methods have not been diagnostic. With the above, it can be concluded that the surgical approach continues to be an option for these patients.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
We would like to thank the Institute of Security and Social Services for State Workers for its support in the publication of the article.
![]() 1, Gutiérrez Vargas HC
1, Gutiérrez Vargas HC ![]() 2, Rodríguez González JS
2, Rodríguez González JS![]() 3, Montano Hernández PA
3, Montano Hernández PA![]() 4, Ortiz Pérez JDC
4, Ortiz Pérez JDC![]() 5, Díaz JM
5, Díaz JM![]() 6 and Ponce Campos SD
6 and Ponce Campos SD![]() *7
*7

