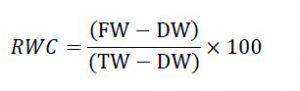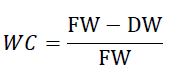Annona squamosa L. is an important fruit crop worldwide, which has become an important crop as a result of its tasty flavor, nutritional value and antioxidant activities. This study aimed at the determination of the phytochemicals and antioxidant compounds present in fruits, seeds and leaves of A. squamosa grown in four sites in Egypt (Menofia, Giza, Alexandria and Mansoura). The highest levels of carbohydrates, folic acid, potassium and calcium of fruits were recorded at Mansoura. The highest contents of protein and sodium were recorded at Alexandria. Flavonoids, total phenols and vitamin C as well as the activity of peroxidase (POX) and polyphenol oxidase (PPO) enzymes were the highest at Menofia. The fruits at Giza had a high content of iron, beside the high enzymatic activity of catalase (CAT) at Giza and also at Alexandria. In seeds, carbohydrates, vitamin C and the activity of CAT, POX and PPO enzymes recorded the highest values at Giza. Flavonoids, total phenol and folic acid were the highest at Menofia, beside the high content of protein was found also as well as at the Alexandria. Vitamin C recorded a high content at Mansoura. In leaves, the highest values of bioactive compounds (flavonoids, total phenol, folic acid, and vitamin C), PPO activity and K+ content were the highest at Giza. The total pigments, protein and Na+ recorded the highest levels at Mansoura. Meanwhile, total carbohydrates as well as CAT and POX activities have a high value at Menofia. Ca2+ and Fe2+ have the highest content at Alexandria. Potassium, sodium, calcium and iron of roots recorded the highest contents at Mansoura followed by Alexandria and Giza.
Plants synthesize a wide variety of natural medicinal compounds and thus attracted considerable interest as the main source for many bioactive metabolites [1]. Plant organs like fruits have potential human health benefits and consequently, they have become increasingly important in human nutrition. The nutritional and health values of fruits are attributed to their high content of bioavailable nutrients and phytochemicals [2].
Recently, the term “functional food” has been introduced to describe foods containing components that induce particular physiological responses along with their nutritional functions and sensory acceptance [3]. It has been advocated that a diet rich in vegetables can lead to a longer and healthier life [4].
The inclusion of fruit and their nutritional derivatives in dietary recommendations requires that they provide appreciable quantities of vitamins, minerals and many other nutrients of health benefits. For instance, folic acid can significantly reduce the risk of coronary heart disease and maintain a healthy body weight [5].
Plant extracts have been used as a source of medicinal compounds for thousands of years.
Custard apple (Annona squamosa L.) is called sugar apple or sweetsop and produces edible fruits that are consumed in many countries. Custard apple is a short (4–6 m high) deciduous tree with many branches [6].The leaves are 5–15 cm long and 2–6 cm wide, with a bluish-green lower surface and a bright green upper surface. Custard apple is the most important tropical fruit and is the most widely distributed among the annonaceous fruits. The plant can withstand rough climatic conditions [7]. The fruits are eaten and are also used in the preparation of beverages and ice creams [8]. The importance of the fruit is attributed to its sweet pulp which has medicinal applications. It also serves as a good source of carbohydrates (23.5%), proteins (1.6%) and minerals (0.9%) [9]. Along with these nutritional constituents, the fruit contains large amounts of vitamins such as folic acid and ascorbic acid as well as minerals such as calcium, phosphorus and iron [10]. In addition, the custard apple is a good source of natural antioxidant compounds, and all the plant parts are used in folk medicine worldwide [11]. The antioxidant activities of fruit extracts, as well as the role of custard apple in preventing oxidative damage, have been reported [11, 12]. Custard apple fruits contain a considerable amount of polyphenolic compounds with antiviral, antimicrobial, and anti-inflammatory activities along with their antioxidant properties [13, 14].
Collection of plant samples
The above-ground parts of A. squamosa including the fruits and some parts of the root were collected from different sites in Egypt i.e., Menofia, Giza, Alexandria and Mansoura during the fruiting season of the year (September–October 2017). In addition, soil samples were collected from each site.
Sample treatments
The mature fruits, leaves and roots were washed with tap water to remove adhered particles. The fruit pulp was manually separated from seeds and peel. An aliquot of the plant parts was used as fresh material and the other part was dried in the oven at 80°C and ground to powder using ceramic mortar and pestle. The powder was sieved with 20 mesh sieves and stored in air-tight polyethylene bags in a desiccator.
Chemical analysis of the soil
Air-dried soil samples were used to determine the soil pH, organic matter, calcium carbonate and total soluble salts as well as chlorides, carbonates, bicarbonates, sulfates, as Na+, K+, Ca2+ and Fe2+. A 1:5 soil:distilled water suspension was used to determine the pH and soluble salts of the soil. The soil suspension was shaken for 1–2 h and filtered through Whatman No. 1 filter paper and the filtrate was used for soil chemical analysis.
Soil reaction (pH) was determined using P.W 9418 pH meter PYE Unicam [15]. Electrical conductivity was monitored using YSI MODEL 33.S.C.T. meter [16]. Calcium carbonate content was determined in the dry soil using the method described by Allen S et al. [17]. Oxidizable organic carbon (as an indication of the total organic matter) was estimated using the Walkley-Black rapid titration method [18]. The total nitrogen was determined by the conventional semi-micro propagation of Kjeldahl method [19] and adopted by Delory E [20]. Total soluble salts were calculated gravimetrically [21]. Sulfates in the soil extract were estimated gravimetrically by using 1% BaCl2 [18]. Carbonates and bicarbonates were determined in the soil extract according to the method described by Jackson M [15]. Estimation of chloride was done by titration against silver nitrate using potassium chromate solution as indicator [18]. The extractable cations Na+, K+, Ca2+ and Fe2+ were assayed in air dried soil using ammonium acetate solution at pH 7 [15]. Na+ and K+ were determined using a flame photometer with a propane burner (type Biologie PHF-808) but Ca2+ and Fe2+ were determined using atomic absorption spectrophotometry (PHF 80B biology Spectrophotometer) [22].
Soil analysis of different sites in the selected four governorates was recorded (Table 1).
Determination of relative water content
Relative water content (RWC) was estimated [23]. Leaf discs were cut from the center of the blade and their fresh weight (FW) was determined. The discs were then floated on distilled water for 4 h, blotted and their fully turgid weight (TW) was recorded. The discs were oven-dried at 80°C till constant weight and their dry weight (DW) was determined. RWC was calculated as:
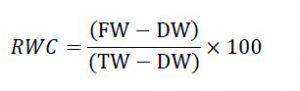
Water content (WC) of other plant organs (fruit, seed and root) was estimated [24] and calculated according to the formula:
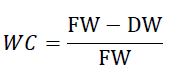
Estimation of photosynthetic pigment
The photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids) were determined by the spectrophotometric method [25] for chlorophylls [26] and carotenoids.
Estimation of carbohydrates
Dried tissue samples (leaves, fruits and seeds) were incubated in 10 cm3 of 80% (v/v) ethanol at 25°C overnight with periodic shaking. The mixture was filtered, and the filtrate was made up to a known volume and kept in the refrigerator for analyses of the different sugar fractions. Glucose was assayed using the o-toluidine procedures [27]. Fructose was estimated using the resorcinol method [28]. Sucrose was estimated using the procedures adopted by Handel E [29]. Total soluble sugars (TSS) content was determined using modification of the procedures [30]. The content of polysaccharides was determined using the method described by Sadasivam S et al. [31]. Total carbohydrates were determined by summation of the TSS and polysaccharides.
Total protein determination
The method of extraction and spectrophotometric determination of protein content in the plant extract was described by Bradford M [32].
Determination of ionic contents
The powdered dried matter was digested in concentrated HNO3 and made up to volume with deionized water [33]. K+ and Na+ concentrations were measured by flame emission spectrophotometer. Ca2+ and Fe2+ concentrations were measured by atomic absorption spectrophotometry (PHF 80B biology Spectrophotometer). Data were calculated as µmol g-1 DW.
Estimation of phenolic compounds
- Determination of total phenolics
The total phenolics content of fruit extract was determined according to the method described by Kosem N et al. [34].
- Determination of total flavonoids
The total flavonoids content was estimated using AlCl3 colorimetric assay [35].
Estimation of vitamins
- Determination of ascorbic acid
Ascorbic acid was quantified according to the method described by Elgailani I et al. [36].
Folic acid was determined according to the spectrophotometric method [37].
Estimation of antioxidant enzymes activity
- Estimation of catalase activity (CAT, EC 1.11.1.6)
CAT activity was determined by slightly modified procedures [38].The reaction mixture contained 1 cm3 of 0.01 M phosphate buffer (pH 7.0), 0.4 cm3 of 0.5 M H2O2 and 0.5 cm3 of the plant extract. The absorbance was measured at 610 nm. One enzyme unit is defined as mmol H2O2/min/g fw.
- Estimation of peroxidase (POX, EC 1.11.1.7)
POX activity was estimated at pH 6 by the increase in absorbance at 420 nm due to the formation of purpurogallin, according to the modified method of Devi P [39].
- Estimation of polyphenol oxidase (PPO, EC 1.14.18.1)
PPO activity was assayed at pH 7 by the increase in absorbance at 420 nm due to the formation of purpurogallin in accordance to the modified method of Devi P [39].
Statistical analysis
The data were statistically analyzed using the procedures reported by Snedecor GW et al. [40] and means were compared using the multiple range tests at the 5% level of probability.
Fruits contribute significantly to the security of food and nutrition. Their important role depends on their typical flavor, taste and nutritional values.
Today, there is an increasing health concerns among consumers about the synthetic foods and their related chemical ingredients. As a result, natural foods and their derivatives are receiving much attention.
Relative water content
Annona species are native to the tropical and subtropical regions like tropical America, eastern Africa and Asia also, but few species are found in the temperate regions [41]. It occurs as an invasive species in some areas. Nowadays it is cultivated in Egypt, Lebanon, Sudan, Saudi Arabia, Oman, Jordan and Palestine [42].
The present results (Table 1), suggest that, Annona squamosa L. grows well in the four sites of Egypt (Menofia, Giza, Alexandria and Mansoura) which exhibited diverse characteristics with some chemical properties. In this concept, Annona species can grow in a wide range of soil types, from sandy soils to clay loam [41].
WC of fruits had the highest value at Menofia followed by Giza, Mansoura and Alexandria. Meanwhile, the WC of seeds was highest at Menofia followed by Alexandria, Mansoura and Giza. In this concept, generally the moisture content of Nigerian sample of Punica granatum seeds is 48.40% indicating that the sample has low moisture content compared to that of Saudi Arabia sample 57.83% [43]. The result is different from those reported by other researchers [44] on juice and seed of the fruits to be 72.6–86.4%. Mahammad M et al. reported 52.3% on seeds, pulp and peel of pears fruits [45]. The low moisture content signifies the higher dry matter yield [46]. The low moisture content of fruits does not favor growth of microorganisms, but aids in the long shelf life period of the produce [47]. On the other word, WC of roots and RWC of leaves showed high elevation at Alexandria and high decreasing at Mansoura. These differences may be due to the differences in the soil properties.
The pH ranged between 7.4 at Alexandria to 8.1 at Mansoura and EC range from 0.15 at Mansoura to 0.67 at Alexandria. In this context, Annona species are grown in calcareous soil [48]. Calcareous soils have high pH (7.5–8.5), high concentration of bicarbonate and generally low organic matter content. This was confirmed with our results (Table 1), except the organic matter that was high in our result. Organic carbon ranged between 12.02% and 41.21% at Giza and Menofia. For total nitrogen, its contents ranged from 0.105–0.134% at Menofia and Alexandria. The highest C/N ratio was recorded at Menofia region where C/N ratio considered as a limiting factor for best growth. A common problem in these soils is an inadequate supply of soluble Fe2+, which results in Fe2+ deficiency in many crops [49] (Table 2).
Photosynthetic pigments
Total chlorophyll and total pigments of A. squamosa leaves were highest at Mansoura followed by Giza, Alexandria and Menofia. The Ch. (a/b) ratio showed non-significant difference between all sites (< 0.05). The carotenoids content of A. squamosa leaves recorded the highest content at Giza followed by Mansoura, Alexandria and Menofia. Chlorophyll plays an important role in plant performance particularly photosynthesis. Remote sensing can provide farmers with location-specific information of chlorophyll content as a representative of crop response to nitrogen application during different growing phases of the plant [50]. Modifications in the main parameters of the photosynthetic process occur in response to environmental changes. These modifications regulate photosynthesis during the day [51] (Figure 1).
Carbohydrate contents
Although A. squamosa is reported to possess important applicable properties due to its insecticidal, antiovulatory and antitumor activities, there is no previous report about the nutritive effect of its fruit pulp [52]. Our present study offers the first scientific report regarding the nutritive value of the fruit pulp of A. squamosa.
- squamosa recorded the highest content of glucose in leaves, seeds and fruits at Menofia and Alexandria, respectively; while the content of glucose was the highest in fruits, seeds and leaves, respectively at Giza. The content of glucose from Mansoura region; in the fruits and leaves was nearly the same and had a higher content than that in seeds.
Fructose content in A. squamosa recorded the highest amount in seeds, fruits and leaves at Menofia and Alexandria. Meanwhile, Giza and Mansoura regions recorded the highest fructose content in fruits followed by seeds and leaves. Sucrose content of fruits was highest at Giza and Mansoura while that of seeds was highest at Menofia and Alexandria. The lowest content of sucrose at all sites was that in leaves.
The highest content of TSS at Giza and Mansoura was found in fruits while the least content was that in leaves. At Menofia, the highest content was found in seeds while the content of TSS was arranged ascending as seeds, leaves and fruits at Alexandria.
Increased carbohydrates content in growing parts of the plant reflects metabolic regulation associated with enhanced enzyme activity which helps plants to withstand environmental conditions and to promote their growth [53].
The highest values of TSS were recorded in plant parts growing in the soil with high sand content which are exposed to water stress due to low soil water holding capacity. Accumulation of high soluble sugar levels have also been demonstrated in shoots of different plant species under stress conditions [54].
The highest content of polysaccharides was found in fruits while the least content was in leaves at Giza and Alexandria and in seeds at Mansoura and Menofia.
Total carbohydrates concentration was in the order fruits > seeds > leaves at Giza and Mansoura. But in the order leaves > fruits > seeds was at Menofia; and seed > fruits > leaves was at Alexandria.
In general the TSS in the fruits at Giza and Menofia were higher than those at Alexandria and Mansoura. Meanwhile, the total carbohydrates in fruits at Mansoura and Giza were higher than at Menofia and Alexandria. The soluble sugar content represented a mix of fructose and glucose (11.75%) in addition to sucrose (9.4%). The fiber combines cellulose, hemicellulose and pectic substances. The degree of fruit ripening does not interfere with these proportions, suggesting that fiber content is determined early ontogeny [41] (Figure 2).
Protein contents
- squamosa at Menofia and Mansoura recorded the highest content of proteins in leaves followed by seeds and fruits. Meanwhile plants growing at Alexandria and Giza recorded the highest protein content in seeds followed by leaves and fruits. In general, the protein content in fruits was higher at Alexandria and Mansoura than Giza and Menofia.
Vitamins contents
- squamosa growing at Menofia, Alexandria and Mansoura recorded the highest content of vitamin C in seeds followed by leaves and fruits. But plants growing at Giza recorded the highest content in leaves followed by fruits and seeds. The fruits at Menofia have a higher content of vitamin C than the other sites. Babu C et al. stated that, A. squamosa is an excellent source of vitamin C [55]. The content of ascorbic acid in custard apple was reported to be in the range of 34–44 mg 100 g-1 [56]. Folic acid content was found in leaves of A. squamosa followed by seeds and fruits at the four regions. Meanwhile the fruits at Mansoura have higher content of folic acid than the other sites (Table 3).
Phenolic contents
- squamosa growing at Menofia, Alexandria and Mansoura recorded the highest content of flavonoids and total phenols in fruits followed by leaves and seeds. But plants growing at Giza recorded the highest content in seeds followed by leaves and fruits. The highest total flavonoid and phenols in fruits contents were found at Menofia.
Enzymes activates
Activities of some antioxidant enzymes revealed that the highest activity of CAT and POX was in leaves of A. squamosa followed by the seeds and fruits at all sites, meanwhile, PPO recorded highest activity in the seeds followed by the leaves and fruits (Table 4).
Ionic contents
Fruits are nutrient suppliers, acting on the metabolism of several functions in humans, and their main nutrients (minerals and vitamins) influence the performance of these functions. Hence, several researchers have conducted studies on the mineral composition of different fruits [57].
Potassium concentration of plant tissue at different sites was highest in roots followed by fruits and leaves except at Alexandria where the highest content was in fruits, followed by leaves and roots. Potassium concentration of fruits was highest at Mansoura. Potassium aids in the fluid balance and nerve impulse transmission within human cells [58].
Sodium concentration of plant tissue at Mansoura was highest in the roots, followed by leaves and fruits. But at the other three sites it was highest in roots followed by fruits and leaves. Fruits had the highest Na+ concentration at Alexandria relative to the other sites.
The (K+/Na+) ratio of plant tissue was highest in fruits followed by leaves and roots at all regions except at Alexandria where the highest content was in leaves followed by fruits and roots. K+/Na+ ratio of plant tissue was highest in fruits followed by leaves and roots at all regions except at Alexandria which has the highest content in leaves followed by fruits and roots. K+/Na+ ratio of fruits was higher at Menofia and Mansoura than the other two sites.
Calcium concentration of plant tissue was highest in leaves followed by roots and fruits at all regions except at Mansoura which has the highest concentration in leaves followed by fruits and roots. Iron concentration of plant tissue was highest in roots followed by fruits and seeds while at Mansoura, it was the highest in leaves followed by roots and fruits. The calcium concentration of fruits was highest at Mansoura.
Calcium is essential for blood clotting and muscle contraction [58]. The fruits at Giza had a rich content of Fe2+. The importance of assure adequate bioavailable dietary Fe2+ arises from the severe consequences associated with iron deficiency and anemia, including reduced immunity and resistance to infection, retardation of body development [59]. Therefore, the Annona fruit becomes important in view of the fact that its regular consumption might ensure adequate supply of iron level into the body. Also, mineral content (Na+, Ca2+, K+ and Fe2+) of the deferent organs of A. squamosa differed in the different sites. The predominant mineral in A. squamosa fruits was K+ which occurred in higher levels than the other fruits such as banana and apple [60].
Roots, leaves, fruits, seeds and bark of custard apple has several applications [61]. The fruits are with high caloric value, minerals and vitamins. Athletes can use it for their high energy content. The seeds have major insecticidal properties and could be used for removing head lice [62] and as a pesticide in agriculture and horticulture. In folk medicine, fruits are effectively used as antidepressant and antioxidant [63] (Table 5).
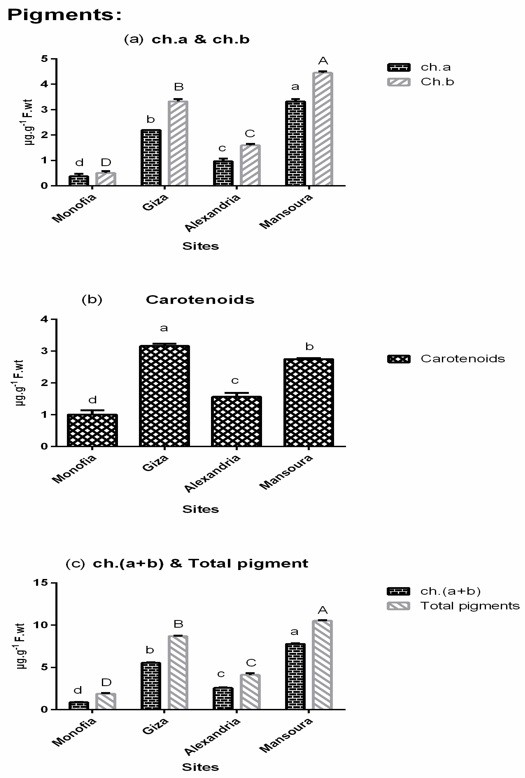
Figure 1: Photosynthetic pigment content in the leaves of Annona squamosa growing at different sites in Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 replicates ± S.E. The columns that share the same letter are not significantly different at P < 0.05.
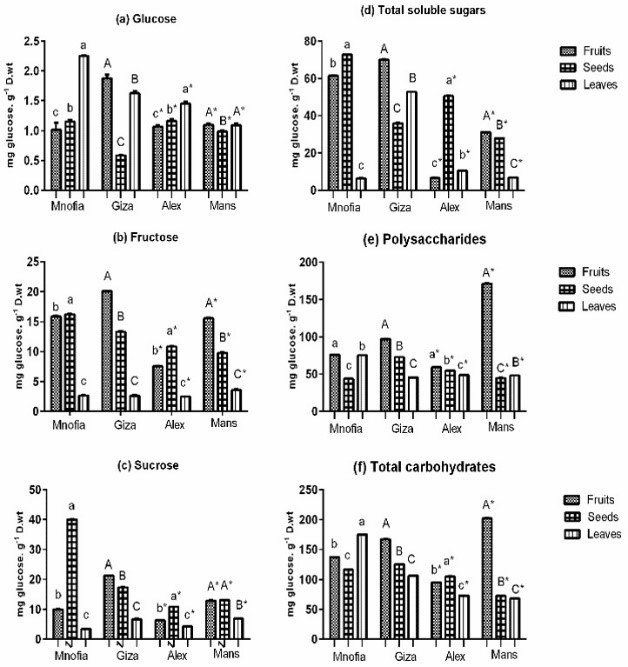
Figure 2: Carbohydrate contents in fruits, seeds and leaves of Annona squamosa, growing at different sites in Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 replicates. The columns that share the same letter are not significantly different at P < 0.05.
| Parameters | Sites |
|---|
| Menofia | Giza | Alexandria | Mansoura |
|---|
| pH | 7.7 | 8.0 | 7.4 | 8.1 |
|---|
| EC (µS/cm) | 0.22 | 0.6 | 0.67 | 0.15 |
| Organic carbon (%) | 41.2 | 12.02 | 13.48 | 15.33 |
| Total nitrogen (%) | 0.105 | 0.128 | 0.134 | 0.118 |
| C/N | 390.17 | 94.14 | 100.34 | 28.02 |
| TSS (%) | 0.664 | 0.165 | 0.116 | 1.87 |
| SO42- (%) | 0.98 | 1.07 | 6.50 | 0.74 |
| CaCO3(%) | 35.5 | 34.5 | 35.5 | 28.0 |
| HCO3− (%) | 0.305 | 0.122 | 0.244 | 0.183 |
| CO3− (%) | Not detected |
| Cl− (%) | 10.0 | 10.0 | 12.5 | 10.0 |
| K+ (µmol g-1 DW) | 1.69 | 1.49 | 0.794 | 3.99 |
| Na+ (µmol g-1 DW) | 9.02 | 11.15 | 16.26 | 29.23 |
| Ca2+(µmol g-1 DW) | 3.66 | 3.40 | 6.55 | 10.96 |
| Fe2+ (µmol g-1 DW) | 0.068 | 0.011 | 0.067 | 0.0004 |
Table 1: Chemical properties of selected soil from different sites in Egypt (Menofia, Giza, Alexandria and Mansoura).
| Organ and measurement | Sites |
|---|
| Menofia | Giza | Alexandria | Mansoura |
|---|
| Root |
|---|
| FW (gm) | 0.04 | 0.03 | 0.04 | 0.02 |
| ± 0.01 | ± 0.03 | ± 0.01 | ± 0.03 |
| DW (gm) | 0.02 | 0.02 | 0.014 | 0.019 |
| ± 0.02 | ± 0.02 | ± 0.01 | ± 0.02 |
| WC (%) | 50.0 | 45.9 | 65.0 | 24.0 |
| ± 0.279b | ± 0.02c | ± 0.01a | ± 0.02d |
| Leaf |
|---|
| FW (gm) | 0.04 | 0.18 | 0.17 | 0.037 |
| ± 0.02 | ± 0.04 | ± 0.01 | ± 0.02 |
| DW (gm) | 0.02 | 0.06 | 0.06 | 0.02 |
| ± 0.01 | ± 0.01 | ± 0.02 | ± 0.01 |
| RWC (%) | 50.0 | 67.5 | 64.7 | 45.9 |
| ± 0.03c | ± 0.04a | ± 0.05b | ± 0.03d |
| Fruit |
|---|
| FW (gm) | 5.3 | 17.4 | 10.0 | 2.96 |
| ± 0.02 | ± 0.02 | ± 0.01 | ± 0.01 |
| DW (gm) | 1.2 | 5.6 | 3.3 | 0.95 |
| ± 0.02 | ± 0.03 | ± 0.1 | ± 0.1 |
| WC (%) | 76.1 | 67.8 | 67.0 | 67.7 |
| ± 0.01a | ± 0.01b | ± 0.2b | ± 0.04b |
| Seed |
|---|
| FW (gm) | 1.0 | 1.14 | 2.0 | 0.51 |
| ± 0.01 | ± 0.01 | ± 0.01 | ± 0.1 |
| DW (gm) | 0.48 | 0.965 | 01.17 | 0.33 |
| ± 0.02 | ± 0.03 | ± 0.02 | ± 0.1 |
| WC (%) | 52.0 | 31.7 | 41.5 | 35.5 |
| ± 0.2a | ± 0.02d | ± 0.3b | ± 0.3c |
Table 2: Fresh weight (gm), dry weight (gm) and relative water content (%) of different Annona squamosa in leaves, fruits, seeds and roots growing at different sites of Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 samples ± S.E. The groups that share the same letter are not significant.
| Organ and site | Protein (mg g-1 FW) | Vitamins |
|---|
| Folic acid ( µg g-1 FW) | Ascorbic acid (mg g-1 FW) |
|---|
| Leaf |
|---|
| Menofia | 13.47 ± 0.11a | 8.12 ± 0.10a | 14.42 ± 0.02b |
| Giza | 6.80 ± 0.11b | 10.47 ± 0.11a | 16.78 ± 0.13b |
| Alexandria | 3.52 ± 0.10b | 10.27 ± 0.10a | 12.43 ± 0.13a |
| Mansoura | 17.26 ± 0.02a | 10.39 ± 0.1a | 11.98 ± 0.20b |
| Fruit |
|---|
| Menofia | 0.737 ± 0.025c | 0.319 ± 0.002c | 1.07 ± 0.002c |
| Giza | 0.754 ± 0.06c | 0.189 ± 0.003c | 0.382 ± 0.001c |
| Alexandria | 1.41 ± 0.05c | 0.175 ± 0.003c | 0.881 ± 0.01b |
| Mansoura | 1.21 ± 0.02c | 0.388 ± 0.001c | 0.602 ± 0.01c |
| Seed |
|---|
| Menofia | 10.50 ± 0.06b | 1.91 ± 0.002b | 36.53 ± 0.03a |
| Giza | 7.87 ± 0.13a | 1.33 ± 0.004b | 48.08 ± 0.11a |
| Alexandria | 10.59 ± 0.13a | 1.33 ± 0.001b | 0.789 ± 0.03c |
| Mansoura | 4.57 ± 0.14b | 0.372 ± 0.001b | 47.87 ± 0.01a |
Table 3: Proteins, folic acid and vitamin C concentrations of leaves, fruits and seeds of Annona squamosa growing at different sites of Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 replicates ± S.E. The means that share the same letter are not significantly different at < 0.05.
| Organ and site | Phenolics | Antioxidant enzyme activities |
|---|
| Flavonoids (µg g-1 DW) | Total phenols (mg gallic acid g-1 DW) | CAT ( mmol H2O2 min-1 g-1 FW) | POX (µmol pyrogallol min-1 g-1 FW) | PPO (µmol pyrogallol min-1 g-1 FW) |
|---|
| Leaf |
|---|
| Menofia | 2.82 | 2.82 | 386.62 | 215.50 | 40.36 |
| ± 0.02c | ± 0.02c | ± 0.12a | ± 0.11a | ± 0.20b |
| Giza | 4.93 | 4.93 | 372.33 | 182.9 | 49.30 |
| ± 0.07c | ± 0.07c | ± 0.12a | ± 0.10a | ± 0.10b |
| Alexandria | 1.08 | 1.08 | 318.42 | 168.50 | 45.50 |
| ± 0.13b | ± 0.13b | ± 3.7a | ± 0.12a | ± 0.03b |
| Mansoura | 1.92 | 1.92 | 368.99 | 145.49 | 48.72 |
| ± 0.20b | ± 0.20b | ± 0.21a | ± 0.11a | ± 0.14b |
| Fruit |
|---|
| Menofia | 9.67 | 9.67 | 11.35 | 34.66 | 19.10 |
| ± 0.10a | ± 0.10a | ± 0.10c | ± 0.21c | ± 0.12c |
| Giza | 1.60 | 1.60 | 18.49 | 10.77 | 11.80 |
| ± 0.04b | ± 0.04b | ± 0.21c | ± 0.11c | ± 0.16c |
| Alexandria | 5.47 | 5.47 | 18.65 | 6.50 | 11.50 |
| ± 0.12a | ± 0.12a | ± 0.12c | ± 0.11c | ± 0.14c |
| Mansoura | 4.60 | 4.60 | 13.18 | 7.53 | 16.00 |
| ± 0.14a | ± 0.14a | ± 0.04c | ± 0.10c | ± 0.03c |
| Seed |
|---|
| Menofia | 5.25 | 5.25 | 113.80 | 75.33 | 70.20 |
| ± 0.11b | ± 0.11b | ± 0.10b | ± 0.0b | ± 0.0a |
| Giza | 0.700 | 0.700 | 145.89 | 92.63 | 75.00 |
| ± 0.03c | ± 0.03c | ± 0.14b | ± 0.01b | ± 0.02a |
| Alexandria | 0.975 | 0.975 | 82.45 | 79.96 | 51.13 |
| ± 0.02c | ± 0.02c | ± 0.11b | ± 0.01b | ± 0.12a |
| Mansoura | 1.10 | 1.10 | 132.76 | 65.30 | 66.20 |
| ± 0.04c | ± 0.04c | ± 0.03b | ± 0.0b | ± 0.22a |
Table 4: Flavonoids and total phenols contents and activities of catalase, peroxidase and polyphenol oxidase in the leaves, fruits and seeds of Annona squamosa growing at different sites of Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 replicates ± S.E. The means that share the same letter are not significantly different at P < 0.05.
| Organ and site | μmol g-1 DW | K+/Na+ ratio |
|---|
| K+ | Na+ | Ca2+ | Fe2+ |
|---|
| Fruit |
|---|
| Menofia | 639.09 | 73.36 | 13.52 | 56.69 | 8.71 |
| ± 0.279a | ± 0.26b | ± 0.13c | ± 0.26b | ± 0.03a |
| Giza | 619.07 | 97.14 | 14.92 | 85.30 | 6.37 |
| ± 0.28a | ± 0.41b | a ± 0.08c | ± 0.10b | ± 0.02a |
| Alexandria | 419.68 | 145.75 | 14.47 | 62.00 | 2.87 |
| ± 0.88a | ± 0.38b | ± 0.06c | ± 0.86b | ± 0.00b |
| Mansoura | 778.77 | 89.58 | 19.47 | 15.02 | 8.69 |
| ± 2.83a | ± 0.13c | ± 0.11b | ± 0.31c | ± 0.03a |
| Leaf |
|---|
| Menofia | 252.47 | 61.2a | 78.51 | 39.90 | 4.12 |
| ± 0.86c | ± 0.27c | ± 0.27a | ± 0.47c | ± 0.00b |
| Giza | 386.98 | 70.01 | 160.29 | 40.33 | 5.52 |
| ± 0.43c | ± 0.05c | ± 0.05a | ± 0.21c | ± 0.01b |
| Alexandria | 315.25 | 85.86 | 171.65 | 49.55 | 3.67 |
| ± 0.48b | ± 0.12c | ± 0.13a | ± 0.21c | ± 0.01a |
| Mansoura | 314.42 | 95.18 | 113.21 | 37.23 | 3.30 |
| ± 0.21c | ± 0.08b | ± 0.08a | ± 0.16a | ± 0.03b |
| Root |
|---|
| Menofia | 460.16 | 218.91 | 74.71 | 75.47 | 2.10 |
| ± 2.20b | ± 0.22a | ± 0.15b | ± 1.8a | ± 0.01c |
| Giza | 393.53 | 212.13 | 88.46 | 171.5 | 1.85 |
| ±1.11b | ± 0.58a | ± 0.30b | ±1.8a | ± 0.01c |
| Alexandria | 298.94 | 235.42 | 73.91 | 81.61 | 1.26 |
| ± 4.33c | ± 0.84a | ± 0.17b | ± 0.24a | ± 0.02c |
| Mansoura | 472.14 | 157.78 | 60.84 | 31.72 | 2.99 |
| ± 11.66b | ± 0.40a | ± 0.13c | ± 0.34b | ± 0.07c |
Table 5: Ionic content of leaves, fruits and roots of Annona squamosa growing at different sites in Egypt (Menofia, Giza, Alexandria and Mansoura). Each value is the mean of 3 replicates ± S.E. The means that share the same letter are not significantly different at P < 0.05.