Abstract
The effect of chemotherapy on the menstrual cycle changes of patients who have undergone cancer treatment, mainly for breast cancer, was studied. For comparison, patients with nasopharyngeal, leukemia, buccal mucosa, and tongue cancers were also included in the study. The average age of the patients was 38. For breast cancer patients, the treatment was mostly a combination of doxorubicin, cyclophosphamide, and docetaxel or paclitaxel. Trastuzumab was also used in combination with other drugs for some patients. Appropriate treatments were given to patients with other cancers. This preliminary study was done with a group of 25 patients. Amenorrhea (stoppage of menses) was observed for about 75% of patients. For those patients for whom menses was restarted, it occurred after 1, 4, or 12 months after complete cessation of chemotherapy. No clear differentiation was observed for breast cancer vs. other cancer patients in the stoppage or restarting of menses with chemotherapy. Induction of amenorrhea after the chemotherapeutic intervention can be considered as a clear indication that the drugs are working as expected. Since the observation was made in patients of child-bearing age, the information could be used for counseling similar patients about the possibility of infertility and teratogenicity associated with chemotherapy. This preliminary study shows that the changes in menstrual characteristics upon chemotherapy in South Indian women are like those reported for the Western population. Induction of amenorrhea and resumption of menses after termination of the chemotherapy, the key parameters evaluated in this study, showed similar trends in both populations.
Keywords
amenorrhea, cancer, chemotherapy, doxorubicin, cyclophosphamide, paclitaxel, docetaxel
Abbreviations
ALL: acute lymphoblastic leukemia, ara-C: cytarabine, AC: doxorubicin and cyclophosphamide, ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine, CAPOX: capecitabine and oxaliplatin
1. Introduction
Cancer is a leading cause of death worldwide, accounting for an estimated 10 million deaths in 2020. According to the data published by the Worldwide Cancer Research Fund, about 18.1 million new cases are reported every year, of which 9.3 million are men and 8.8 million are women [1]. In India, cancer cases are more common among women than men. Breast, cervical, ovarian, and uterine cancer account for more than 70% of cancers among women in India [2]. It is also reported that the peak age for the onset of breast and ovarian cancer in India (45–50 years) is a decade younger than the peak age in high-income countries (more than 60 years), which is attributed mainly to genetic and environmental factors, high-fat diet, late marriage, fewer children, inadequate breastfeeding, etc. [3, 4]. There are many studies linking cancer in women, particularly hormone-dependent cancers like breast cancer, ovarian cancer, uterine cancer, and cervical cancer, to the changes in menstrual patterns like the cycle length, frequency, abnormal cycles, heavy bleeding, etc. [5–8]. It is also known that chemotherapy for cancer can also cause menstrual changes [9].
The effect of various cancers on the menstrual pattern of women was the subject of many studies. Now, there is enough evidence to demonstrate a link between hormone-dependent cancers and the menstrual pattern [5–6]. In one study to find out the relationship between breast cancer risk and menstrual changes, the authors conducted a mail survey among 997 women who had recorded menstrual events prospectively over as many as 50 years, beginning in 1934 [10]. Compared with women with a median menstrual cycle length of 26–29 days, women who had cycles of extreme length at ages 25–29 years had a nearly two-fold increase in the incidence of breast cancer. Another study showed that having a higher number of lifetime ovulatory cycles and starting periods earlier, at a younger age, increases the risk of death after a diagnosis of ovarian cancer [11]. Most women with endometrial cancer (uterine cancer) have early symptoms, which include irregular menstrual bleeding, spotting, and bleeding between menstrual periods [7]. In women, many cancers are associated with an imbalance of the sex hormones estrogen and progesterone, which in turn can alter the menstrual cycle pattern. These hormones are produced in the ovaries in premenopausal women and by some other tissues, including fat and skin, in both premenopausal and post-menopausal women. Estrogen promotes the development and maintenance of female sex characteristics and the growth of long bones, while progesterone plays a role in the menstrual cycle and pregnancy.
Many cancer chemotherapies are associated with decreased hormone production or binding to their receptors and can lead to hormonal imbalance, causing major changes in their function, particularly the menstrual cycle characteristics like the cycle length, frequency, abnormal cycles, etc. It may also be associated with hot flashes, night sweats, and vaginal dryness [12–14]. The treatments can reduce similar abnormalities induced by cancer but can also induce similar changes due to the effect on estrogen or progesterone production/binding to the receptors. Some cancer treatments can affect the normal functioning of the ovaries. This can sometimes lead to infertility and an earlier-than-expected onset of menopause, even at a young age. There are many studies that link the chemotherapeutic regimen of paclitaxel/docetaxel with cyclophosphamide and doxorubicin to amenorrhea, the stoppage of menses [15, 16]. There are also reports of heavy bleeding associated with chemotherapeutic treatment for hematological cancers [17, 18]. So, the literature available so far indicates a strong link between the effects of chemotherapy on menstrual characteristics of premenopausal women.
A study on the effect of chemotherapy on menstrual characteristics will be useful because it may indicate the efficacy of the chemotherapeutic agents to cure cancer and the toxicity effects that may affect ovarian function. It will also be useful in predicting the fertility of pre-menopausal women taking chemotherapeutic treatment. Most of the data available so far on the chemotherapy effect on the menstrual pattern is from the Western population and may be quite different for the Indian population because the menstrual pattern is also known to be dependent on many other factors like eating habits, exercise, use of oral contraceptives, starting age of menses, etc. In this paper, we report the results of a preliminary study among female cancer patients undergoing chemotherapeutic treatment for various cancers, mainly for breast cancer, conducted to see whether the treatment has a definitive relationship with menstrual patterns and associated changes among premenopausal women, mostly from South Indian population.
2. Materials and Methods
In this study, data was collected by conducting a survey among the women patients who undergone chemotherapy treatment for cancer (mainly breast cancer) at the Medical Oncology Department, Father Muller Medical College, Mangalore, India. These patients were from South India and the majority of them were breast cancer patients. The details of the patient population in the study are given in the figure below (Figure 1).
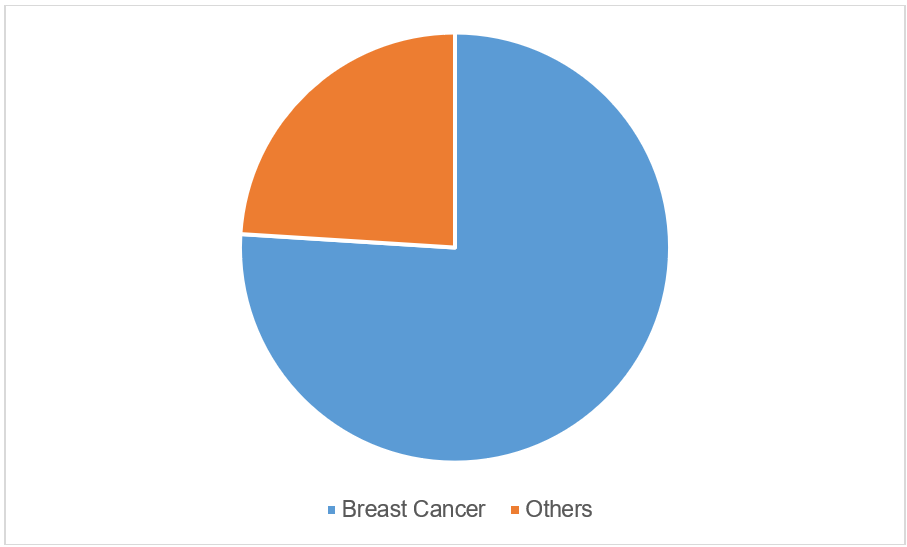 Figure 1: Patients included in the study. Others: acute lymphoblastic leukemia (ALL), cancer of the tongue, nasopharyngeal cancer, left buccal cancer, colon cancer, and non-Hodgkin’s lymphoma.
Figure 1: Patients included in the study. Others: acute lymphoblastic leukemia (ALL), cancer of the tongue, nasopharyngeal cancer, left buccal cancer, colon cancer, and non-Hodgkin’s lymphoma.
Researchers interacted with the patients and collected information about the menstrual pattern and related details. The information was recorded after informing the patients that the data would be used for research purposes only. Data included information about the starting age of menses, duration of the menstrual cycle, any irregularity in the period, whether menses were restarted after treatment, and if so, after how many days or months, etc. In addition, all the treatment details like cancer type, stage of cancer at the time of treatment, chemotherapeutic agents used, number of treatment cycles, etc. were also collected. Data was analyzed using the Microsoft Excel program, and bar graphs were generated to demonstrate the patient population with or without treatment-related effects.
2.1 Inclusion and exclusion criteria
Only pre-menopausal women aged between 16–45 years of age taking chemotherapy in the first-line setting were included in the study. Women who already had menopause, women who have undergone hysterectomy or oophorectomy, and women who have received hormonal therapy for cancer were not included in the study. Women who have received radiotherapy to the abdomen or pelvic region were also excluded because the radiation to the abdomen or pelvic region may affect the menstrual pattern on its own, and hence, may be difficult to differentiate the effect from chemotherapy.
2.2 Treatment
A combination of doxorubicin, cyclophosphamide, and docetaxel or paclitaxel was the treatment given for breast cancer. Trastuzumab was also included in the treatment for some patients. Treatments given for all cancers included in the study are given in the table (Table 1).
| Cancer type | Chemotherapy |
| Breast cancer | Adj. AC + paclitaxel +trastuzumab |
| Adj. AC + paclitaxel+ carboplatin |
| Adj. AC + paclitaxel |
| Acute lymphoblastic leukemia | Endoxan + ara-C |
| Cancer of tongue | Paclitaxel + cisplatin |
| Colon | Adj. CALPOX |
| Nasopharyngeal | Docetaxel + cisplatin + FU |
| Hodgkin’s lymphoma | ABVD/ABD |
| Left buccal | Docetaxel + cisplatin |
Table 1: Different cancer types in this study and the treatment provided for each cancer.
2.3 Data analysis
After collecting the data from 25 patients, it was analyzed to find the overall effect of treatment on various parameters like duration of the menstrual cycle, abnormal bleeding, cancer stage when treatment was started, early onset of menopause, and restarting of the period after treatment to answer the following questions.
- Is there any definite link between menstrual patterns and chemotherapy for cancer among South Indian patients?
- In which type of cancer does the chemotherapy significantly affect the menstrual pattern?
- Which chemotherapeutic drug has a significant effect on menstrual patterns?
- Does the cancer stage have any effect on chemotherapy-related effects on menstrual patterns?
- Does the age of the patient have any effect on chemotherapy-related effects on the menstrual pattern?
- Are there any other observable changes associated with chemotherapy (excessive bleeding, irregular bleeding, pain, etc.)?
3. Results and Discussion
Induction of amenorrhea and related effects associated with the treatment of cancer patients using chemotherapeutic agents doxorubicin, cyclophosphamide, and Taxol-based anticancer drug paclitaxel or docetaxel was the primary theme of this study (Table 2). The key finding from our study is that amenorrhea was observed in 82% of patients who were treated with such therapy. Since most of the patients (76%) in the study group were treated for breast cancer, a combination of doxorubicin, cyclophosphamide, and paclitaxel/docetaxel was part of the standard treatment regimen. Chemotherapeutic treatment of cancer patients with such a drug combination is known to induce amenorrhea in premenopausal women [15, 19, 20]. Swain et al. [21], who studied the induction of amenorrhea associated with the treatment regimen of doxorubicin, cyclophosphamide, and docetaxel/paclitaxel, have reported amenorrhea for ~83% of patients for at least 6 months. In our study, we observed amenorrhea in approximately 75% of patients for at least one year. The biological function that is affected by these drugs was studied in some detail earlier. It is hypothesized that the drug may bind the breast cancer receptors estrogen and progesterone and ovarian follicles to induce amenorrhea [22].
| Cancer type | Amenorrhea was observed | Menses resumed |
| Breast cancer | Yes – 95% | No – 72% |
| Acute lymphoblastic leukemia | Yes | No |
| Cancer of tongue | No | NA |
| Colon | No | NA |
| Nasopharyngeal | NA | NA |
| Hodgkin’s lymphoma | Yes | Yes |
| Left buccal | Yes | No |
Table 2: Induction of amenorrhea and resumption of menses of patients treated with chemotherapy for different types of cancers.
Resumption of menses after amenorrhea is another important factor that is associated with chemotherapy. This is particularly important for women patients who are of childbearing age. It is known that the incidence of amenorrhea increases with age. Jacobson et al. [23] studied the resumption of menses after amenorrhea and observed that it is correlated with the age of the patients. In patients ≤35 years, menses were reported to have resumed for almost 70% of women. In our study, we found that menses resumed for only 30% of women for whom data was available. This can be attributed to the higher age of patients in our study and the lower period of post-treatment observation (1 year as compared to 2 years). The figure (Figure 2) below shows a clear trend of amenorrhea induction and resumption of menses with chemotherapy in our study group.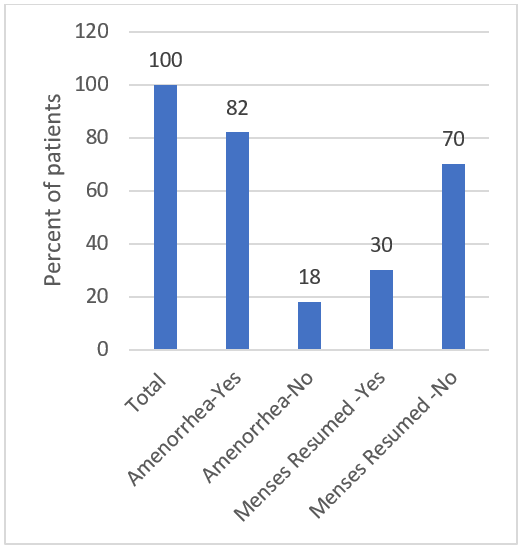 Figure 2: Status of induction of amenorrhea and resumption of menses in chemotherapy-treated cancer patients. Data is represented as the percentage of patients and was normalized for the number of patients for whom data was available.
Figure 2: Status of induction of amenorrhea and resumption of menses in chemotherapy-treated cancer patients. Data is represented as the percentage of patients and was normalized for the number of patients for whom data was available.
Amenorrhea was observed for an acute lymphoblastic leukemia (ALL) patient of 16 years of age, and menses were not restarted, while for the only non-Hodgkin’s lymphoma patient of 22 years, amenorrhea was induced, but menses were restarted in a month. So, we could not make a conclusion on the age effect on chemotherapy-induced amenorrhea. However, it is to be noted that these patients were treated with different treatment regimens (doxorubicin, bleomycin, vinblastine, dacarbazine for non-Hodgkin’s lymphoma, and endoxan and ara-C for ALL). Boltežar et al. [24], who studied the chemotherapeutic effect, concluded that amenorrhea is not a significant factor in non-Hodgkin’s lymphoma treatment, probably because the drugs used do not have effects similar to that observed for breast cancer.
To understand how fast the chemotherapeutic treatment can affect the menstrual characteristic, induction of amenorrhea was monitored with each treatment cycle. The data was available for a few patients, and it was noted that for almost half of them, amenorrhea started after the first cycle itself, and for the rest, it was observed after 2 cycles. We also looked at whether the chemotherapeutic treatment affected the menstrual pattern. Of the patients for whom data is available, all of them had regular periods (25/28 days), and the menses were stopped for most of them on chemotherapeutic treatment. However, for two patients, menses were not stopped, and for one patient, it became irregular. No issues of bleeding were reported by any patients.
The only colon cancer patient who was treated with adjuvant capecitabine and oxaliplatin (CAPOX) did not experience any change in menstrual pattern, and no amenorrhea was observed. Wan et al. [16], who studied the effect of CAPOX treatment, reported that they found amenorrhea only in about 4% of patients. Based on this study, breast cancer drugs are suggested to be included in the treatment of ovarian cancer to suppress ovarian function.
4. Conclusion
The preliminary results of this ongoing study showed a very good correlation between the chemotherapeutic treatment for cancers and menstrual cycle changes in pre-menopausal patients of the South Indian population, in line with the observed data for the Western population. For most of the treated patients, amenorrhea was induced on treatment, and menses were not resumed for the one-year observational period. These results have a predictive value on the effect of treatment and may help in understanding any toxicity associated with the treatment. It will also help in counseling patients of childbearing age about the potential effects associated with pregnancy upon chemotherapeutic treatment.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding Statement
The authors would like to mention that no funding was received from any agencies for carrying out the work presented in this article.
Acknowledgments
The authors would like to acknowledge Fr. Muller Medical College for all the support provided during the data collection and manuscript preparation.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
- Sathishkumar K, Chaturvedi M, Das P, et al. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J Med Res. 2022;156(4&5):598-607.
- McDonald JA, Rao R, Gibbons M, et al. Symposium report: breast cancer in India-trends, environmental exposures and clinical implications. Cancer Causes Control. 2021;32(6):567-75.
- Giri K, Mehta A, Ambatipudi K. In search of the altering salivary proteome in metastatic breast and ovarian cancers. FASEB Bioadv. 2019;1(3):191-207.
- Terry KL, Willett WC, Rich-Edwards JW, et al. Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1509-513.
- Titus-Ernstoff L, Perez K, Cramer DW, et al. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84(5):714-21.
- Modugno F, Ness RB, Chen C, et al. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2840-847.
- Varghese H, Qureshi MAS, Ronghe A, et al. Prevalence and Sociodemographic Covariates of Cervical Cancer and Its Association With Menstrual Irregularities: Findings From the 2017 National Inpatient Sample Database. Cureus. 2021;13(10):e18855.
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145(1):113-28.
- Grant CS, Ingle JN, Suman VJ, et al. Menstrual cycle and surgical treatment of breast cancer: findings from the NCCTG N9431 study. J Clin Oncol. 2009;27(22):3620-626.
- Yang HP, Murphy KR, Pfeiffer RM, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol. 2016;183(9):800-14.
- Partridge A, Gelber S, Gelber RD, et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43(11):1646-653.
- Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102-111.
- Tiong V, Rozita AM, Taib NA, et al. Incidence of chemotherapy-induced ovarian failure in premenopausal women undergoing chemotherapy for breast cancer. World J Surg. 2014;38(9):2288-296.
- Vanhuyse M, Fournier C, Bonneterre J. Chemotherapy-induced amenorrhea: influence on disease-free survival and overall survival in receptor-positive premenopausal early breast cancer patients. Ann Oncol. 2005;16(8):1283-288.
- Wan J, Gai Y, Li G, et al. Incidence of chemotherapy- and chemoradiotherapy-induced amenorrhea in premenopausal women with stage II/III colorectal cancer. Clin Colorectal Cancer. 2015;14(1):31-4.
- Nebgen DR, Rhodes HE, Hartman C, et al. Abnormal Uterine Bleeding as the Presenting Symptom of Hematologic Cancer. Obstet Gynecol. 2016;128(2):357-63.
- Gao A, Zhang L, Zhong D. Chemotherapy-induced thrombocytopenia: literature review. Discov Oncol. 2023;14(1):10.
- Woods BS, Sideris E, Sydes MR, et al. Addition of Docetaxel to First-line Long-term Hormone Therapy in Prostate Cancer (STAMPEDE): Modelling to Estimate Long-term Survival, Quality-adjusted Survival, and Cost-effectiveness. Eur Urol Oncol. 2018;1(6):449-58.
- Wang Y, Li Y, Liang J, et al. Chemotherapy-Induced Amenorrhea and Its Prognostic Significance in Premenopausal Women With Breast Cancer: An Updated Meta-Analysis. Front Oncol. 2022;12:859974.
- Swain SM, Land SR, Ritter MW, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113(2):315-20.
- Andre F, Broglio K, Pusztai L, et al. Estrogen receptor expression and docetaxel efficacy in patients with metastatic breast cancer: a pooled analysis of four randomized trials. Oncologist. 2010;15(5):476-83.
- Jacobson MH, Mertens AC, Spencer JB, et al. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril. 2016;105(3):765-72.e4.
- Boltežar L, Pintarić K, Jezeršek Novaković B. Fertility in young patients following treatment for Hodgkin’s lymphoma: a single center survey. J Assist Reprod Genet. 2016;33(3):325-33.


