Abstract
Background and Purpose: The pharmacotherapy options in patients with gestational diabetes mellitus (GDM) are insulin or oral antihyperglycemic agents. Insulin is the preferred medication for treating hyperglycemia, but in recent years, metformin has been increasingly used in the treatment of GDM.
Aim: The aim is to assess the efficacy of different treatments (insulin and metformin) in women with GDM.
Methods: Screening for GDM was performed in 2422 pregnant women and revealed GDM in 119 women [75 g oral glucose tolerance test (OGTT) was performed at 24–28 weeks of gestation]. All patients started treatment at 24–29 weeks of gestation. The patients were divided into two groups (Gr.): Gr. 1 had 68 patients treated with diet and insulin therapy. Gr. 2 had 51 patients treated with diet and metformin.
Results: In the 2nd trimester, HbA1c (%) levels for Gr. 1 and Gr. 2 were 6.7 (0.05) and 6.4 (0.6), respectively. By term, HbA1c levels statistically decreased in both groups, but we did not find a statistical difference between the groups. Women from Gr. 2 gained less weight compared to Gr. 1 (1.89 ± 3.88 vs. 4.53 ± 3.67 kg; P = 0.003). In Gr. 1, the percentage of preeclampsia was 2.9%, and in Gr. 2, 3.9% (P = 0.7773, OR – 1.33). We did not find a statistical difference between the groups. The incidence of preterm delivery before 37 weeks of gestation in Gr. 1 was lower than in Gr. 2 (P = 0.7311, OR – 1.33), and we also did not find a statistical difference between the groups. Perinatal mortality was observed in Gr. 1 – 1.4% and in Gr. 2 – 1.9% (P = 0.8402, OR – 1.33). In both groups, we observed a high percentage of cesarean section (Gr. 1 – 32.3% and Gr. 2 – 29.4% (P = 0.7651, OR -1.0909), but we did not find a statistical difference between the groups. In both groups, the percentage of macrosomia was high, despite good glycemic control maintained through pregnancies: 20.0% and 23.0% for Gr. 1 and Gr. 2, respectively (P = 0.9236, OR – 1.0256), and again no statistical difference was found between the groups. Percent of neonatal hypoglycemia was lower in Gr. 2 (1.9%) than in Gr. 1 (4.41%) (P = 0.9236, OR – 1.0256). Percent of respiratory distress syndrome was 2.94% and 3.92% for Gr. 1 and Gr. 2, respectively (P = 0.9694, OR – 0.9893), with no statistical difference between the groups.
Conclusion: We did not find differences between patients treated with diet and insulin therapy and patients treated with diet and metformin. The percentage of preeclampsia, preterm delivery, macrosomia, and perinatal death were similar in both groups; only maternal weight gain was lower in the metformin group.
Keywords
GDM, preeclampsia, preterm delivery, perinatal mortality, macrosomia
Abbreviations
GDM: gestational diabetes mellitus, OGTT: oral glucose tolerance test, Gr.: group, IDF: International Diabetes Federation, ADA: American Diabetes Association, WHO: World Health Organization, LGA: large for gestational age, IADPSG: International Association of Diabetes and Pregnancy Study Groups, FIGO: International Federation of Gynecology and Obstetrics, FPG: fasting plasma glucose, SBGM: self-blood glucose monitoring, TSH: thyroid-stimulating hormone, BMI: body mass index, CGM: continuous glucose monitoring, SMD: standardized mean difference, OR: odds ratio, CIs: confidence intervals
1. Introduction
Gestational diabetes mellitus (GDM) is a widespread complication of pregnancy, defined as glucose intolerance of various severity that is first diagnosed during pregnancy [1]. According to the International Diabetes Federation (IDF) in 2019, “every sixth newborn was born to a mother with hyperglycemia” [2]. In a systematic review published in 2012 [3], the prevalence of GDM varied according to diagnostic criteria: The American Diabetes Association (ADA) reported the prevalence of GDM at 2–19% [4], and according to the World Health Organization (WHO), the prevalence of GDM is 2–24.5% [5]. Women with GDM, detected during pregnancy, are at greater risk of adverse pregnancy outcomes. These include high blood pressure and a baby large for gestational age (LGA), which can make a normal birth difficult. Detection of hyperglycemia during pregnancy and the presence of normoglycemia during pregnancy can reduce these risks. The pharmacotherapy options in patients with GDM who require pharmacotherapy are insulin or oral antihyperglycemic agents (metformin or glyburide). Some meta-analysis studies have shown that both oral antihyperglycemic agents and insulin therapy can improve pregnancy outcomes in patients with GDM or type 2 diabetes [6].
The aim of the study that was carried out at our center was to assess the efficacy of different treatment options (insulin and metformin) in women with GDM.
2. Methods
The study was carried out at the National Center for Diabetes Research in Tbilisi, Georgia, in 2015–2022. Screening for GDM was performed in 2,422 pregnant women, and GDM was revealed in 119 of them. The “one-step” 75 g oral glucose tolerance test (OGTT), derived from the International Association of Diabetes and Pregnancy Study Groups (IADPSG) [7], the International Federation of Gynecology and Obstetrics (FIGO) [8] and the WHO [5] criteria were used. OGTT was performed at 24–28 weeks of gestation. The test was performed after an 8-hour fasting period; fasting plasma glucose (FPG) was measured, then patients were advised to drink a solution that contained 75 g of dry glucose powder dissolved in 250 ml of lukewarm water, then blood samples were drowned 1 and 2 hours post load. If a plasma glucose value is above or equal to 92 mg/dL (5.1 mmol/L) fasting, 180 mg/dL (10.0 mmol/L) 1 hour, and 153 mg/dL (8.5 mmol/L) 2 hours, GDM is suspected. Three patients were referred to our center by antenatal clinics with hyperglycemia, so no OGGT was performed on them. One patient was on the 18th week of gestation (FPG – 182 mg/dL), the second on the 31st week (FPG -138 mg/dL), and the third on the 30th week of gestation (FPG – 149 mg/dL). As in all three patients, FPG levels were above 92 mg/dL (5.1 mmol/L), diagnosis of GDM was made before they were admitted to our center.
After the diagnosis of GDM was confirmed, all necessary clinical and laboratory tests and investigations were performed. Follow-up throughout the pregnancy: adjustment of daily calorie intake – once in trimester; correction of intensive insulin therapy or metformin dose based on the self-blood glucose monitoring (SBGM) – once in a week; registration of hypoglycemia episodes – every visit; HbA1c – every 4 weeks; SBGM – 7–6 times a day; microalbuminuria – every two weeks; creatinine – once in trimester; thyroid-stimulating hormone (TSH) and thyroid peroxidase antibodies – once in trimester; blood pressure control – every visit (antihypertensive therapy was initiated if blood pressure ≥135/85 mmHg); ultrasound examination, cardio monitoring of a fetus, obstetrical/gynecologic follow-up; consultations of a neurologist, cardiologist and nephrologist – once in trimester; low-dose aspirin 75 mg/day from the end of the first trimester.
The patients were divided into two groups (Gr.): Gr. 1 had 68 patients treated with diet and insulin, and Gr. 2 had 51 patients treated with diet and metformin. All patients had their first pregnancy.
Lifestyle behavior change was an important component in the management of GDM in our patients. Treatment started with medical nutrition therapy, physical activity, and weight management, depending on pre-gestational weight. Calorie intake for each patient was calculated individually and depended on the pre-pregnancy body mass index (BMI). BMI of 20–29 kg/m² was registered in 86 of our patients, and were recommended a calorie intake of 30–32 kcal/per kg of body weight/day. BMI of 33 patients was 29.5–33 kg/m² and were recommended 24–25 kcal/per kg of body weight/day. Calories were divided as follows: 175 g of carbohydrates, 71 g of protein, and 28 g of fiber. Concerning fats, it was recommended to increase the amount of monounsaturated and polyunsaturated fats, limit saturated fats, and exclude trans fats [9, 10]. We recommended effective exercise (aerobic, resistance, or both), with a duration of 20–50 min/day (2–7 days/per week of moderate-intensity exercise) [11].
Glucose monitoring was aimed at the targets recommended by the Fifth International Workshop-Conference on Gestational Diabetes Mellitus [12]. Following glycemic targets were implemented: fasting BG – 60–90 mg/dL (3.3–5.0 mmol/L), postprandial 1-hour BG < 140 mg/dL (< 7.7 mmol/L), postprandial 2-hours BG < 120 mg/dL (< 6.6 mmol/L), and BG before each meal – 75–105 mg/dL (4.1–5.8 mmol/L). According to the ADA recommendations, the A1C target of < 6% (42 mmol/mol) is optimal during pregnancy if it can be achieved without significant hypoglycemia [13]. SBGM was recommended for all our patients, and blood glucose was measured – fasting and postprandial (1- and 2-hours/2–3 times a day); eight of our women were using continuous glucose monitoring (CGM) systems [14].
At the start (18-31 gestation weeks), all patients were on diet and exercise for a minimum period of one week, and if their glycemic control was unsatisfactory, medication-based treatment was initiated. Based on the FIGO recommendations [8], insulin therapy was initiated if the age of gestation at GDM diagnosis was < 20 or > 30 weeks; FPG level was > 110 mg/dL (6.1 mmol/L); 1-hour postprandial glucose was > 140 mg/dL (7.7 mmol/L); pregnancy weight gain was > 12 kg.
Insulin types and regimens were individualized [15, 16]. Rapid-acting and long-acting insulin analogues were used. The starting dose of long-acting insulin analogue (glargine) was 0.2 UI/kg/24 h at bedtime. To achieve adequate glycemic control, the doses were adjusted every 3 days. If preprandial glucose levels were normal and postprandial ones were high, rapid-acting insulin analogue (glulizin -0.1 UI/kg/meal) was added before each meal.
In our study, we gave initial metformin dose – 1000 mg/day (500 mg 2 times a day) and if adequate control (fasting – 60–90 mg/dL (3.3–5.0 mmol/L), postprandial 1-hour < 140 mg/dL (< 7.7 mmol/L), postprandial 2-hours < 120 mg/dL (< 6.6 mmol/L), before a meal – 75–105 mg/dL (4.1–5.8 mmol/L)) was not achieved, the dose was raised the next week to 1500 mg/day (500 mg 3 times a day), the maximal dose was 2000 mg/day. Patients who did not achieve satisfactory glycemic control with metformin received supplemental insulin analogues. Glargine was initiated in 4 women in the 30–32 weeks of gestation, while in all cases, metformin was continued at the maximal dose.
The main aim of GDM management is to achieve normoglycemia during all courses of pregnancy since numerous studies indicate that normal fetal growth can be achieved if in patients with GDM, glucose levels during pregnancy are close to normal [17]. In our study, the women were followed up until they delivered and then postpartum.
Definition of some terms: Preterm delivery – birth before 37 weeks of gestation; Preeclampsia – hypertension, edema, and proteinuria after 20 weeks of gestation; Neonatal hypoglycemia – a plasma glucose level of less than 40 mg/dL (2.2 mmol/L) in the first 48 hours of life; Macrosomia – neonatal weight ≥4,000 g.
Statistical analysis: The mean and standard deviation for continuous variables were used to calculate the standardized mean difference (SMD). The odds ratio (OR) was calculated for dichotomous variables with 95% confidence intervals (CIs).
Primary outcomes include maternal glycemic control (HbA1c) during pregnancy and maternal weight gain at birth. Secondary outcomes included incidence of preeclampsia, preterm delivery, cesarean section, macrosomia, LGA infants (large for gestational age infant, defined as birth weight ≥90th percentile for gestational age and infant’s sex), malformations, respiratory distress syndrome, and perinatal mortality.
3. Results
There were no statistically significant differences between Gr. 1 and 2 in terms of maternal age, pre-pregnancy BMI, gestational age, and HbA1c levels at baseline (Table 1).
At the start of the treatment, HbA1c levels for Gr. 1 and 2 were 6.5% (0.5) and 6.4% (0.5), respectively. In the 3rd trimester, HbA1c levels statistically decreased in both groups: Gr. 1 – 6.01% (0.5) and Gr. 2 – 5.9% (0.6), while no statistical difference was observed between the groups (Р = 0.3239) (Figure 1).
| Gr. 1 – 68 | Gr. 2 – 51 | P (Gr. 1-2) |
| Age (years) | 26.2 ± 5.1 | 25.9 ± 5.3 | 0.967536 |
| Pre-pregnancy BMI (kg/m2) | 25.6 ± 4.9 | 25.9 ± 5.1 | 0.921329 |
| Gestation age at the beginning of treatment (weeks) | 25.4 ± 4.36 | 25.9 ± 3.98 | 0.932648 |
| HbA1c (%) | 6.5 ± 0.5 | 6.4 ± 0.5 | 0.3876 |
| Microalbuminuria (%) | 5.8 | 5.9 | |
Table 1: Clinical data of 119 women with GDM.
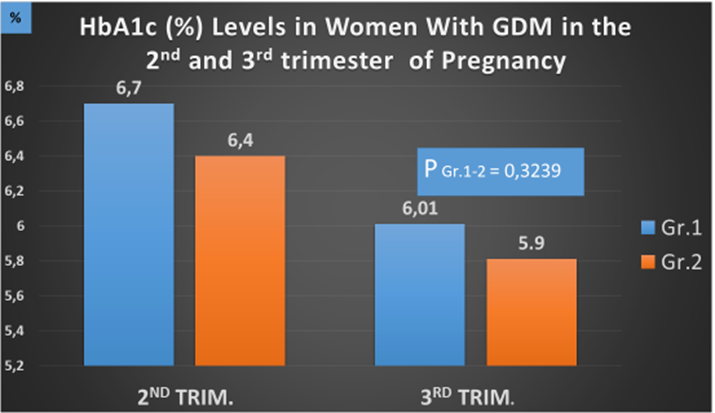 Figure 1: HbA1c levels in women with GDM in the 2nd and 3rd trimester of pregnancy.
Figure 1: HbA1c levels in women with GDM in the 2nd and 3rd trimester of pregnancy.
From the start of the treatment to before delivery (36–39 gestational weeks), women from Gr. 2 (metformin-treated group) gained less weight compared to women from Gr. 1 (insulin-treated group) (1.89 ± 3.88 vs. 4.53 ± 3.67 kg; P = 0.003). The same data were obtained in a multicenter, open-label, parallel arms, randomized clinical trial (The Metformin for Gestational Diabetes -2021), the studies enrolled women with gestational diabetes who needed pharmacologic treatment [18]. In support of this, recent meta-analyses have shown that weight gain in GDM patients treated with metformin during pregnancy was significantly lower than in those treated with insulin [6, 18, 19].
We observed preeclampsia in patients from both groups. In Gr. 1 patients, the percentage of preeclampsia was 2.9%, and in Gr. 2, it was 3.9% (P = 0.7773, OR – 1.33). The insulin-treated group had a lower incidence of preeclampsia compared to the metformin-treated group, but the difference was not significant. Preeclampsia in women with GDM was also observed in other studies. Some studies identify different causes of preeclampsia, as the HAPO study showed that the occurrence of preeclampsia is positively associated with blood glucose levels [20]. In the study conducted by Catalano et al. [21], the development of preeclampsia in women with GDM was directly associated with overweight and obesity. The same results were obtained in a Swedish retrospective cohort study [22], but another retrospective cohort study showed that obesity is not associated with the occurrence of preeclampsia in women with GDM [23]. Some retrospective cohort studies have shown that gestational diabetes, regardless of other causes, leads to the development of preeclampsia [23–26]. Results obtained in our study have been confirmed by the randomized controlled trial conducted in New Zealand and Australia, the incidence of preeclampsia in the metformin group was lower than in the insulin group, and the difference was not statistically significant [27]. Other studies have confirmed the fact that the appointment of metformin during pregnancy had no effect on the incidence of preeclampsia [8, 28]. However, in the metformin group, weight gain after the start of treatment was less.
In our study, the percentage of preterm deliveries was lower in Gr. 1 – 4.41% compared to Gr. 2 – 5.88% (P = 0.7311, OR -1.33), though there was no statistical difference between the groups. The incidence of preterm delivery before 37 weeks of gestation in the insulin-treated group (4.41%) was lower than that in the metformin-treated group (5.88%), but the difference was not statistically significant (P = 0.7311, OR -1.33) (Table 2 and Figure 2). In our study, the use of metformin did not induce an increase in the incidence of preterm delivery before 37 weeks of gestation. However, results obtained in other studies have shown that gestational diabetes is associated with an increased risk of preterm delivery, birth trauma, and respiratory distress syndrome [29, 30].
In our study, perinatal mortality was observed in both groups. It comprised 1.4% in Gr. 1 and 1.9% in Gr. 2 (P = 0.8402, OR – 1.33), and again, no statistical difference was found between the groups (Figure 2).
Stillbirth was registered only in Gr. 1 and its rate was 1.4%, while neonatal death only in Gr. 2 (1.9%) (Table 3). A retrospective study in the United States (more than 4 million women) showed that the risk of stillbirth between 36 and 42 weeks of gestation was higher in women with GDM than in women without GDM (17.1 vs. 12.7 per 10,000 births) (RR 1.34; 95% CI 1.2-1.5) [31].
Percent of cesarean section was high in both groups, Gr. 1 – 32.3% and Gr. 2 – 29.4% (P = 0.7651, OR -1.0909). However, we did not find a statistical difference between the groups (Table 2). The newborn’s birth weight was 3495 ± 493. 5 g in Gr. 1 and 3570 ± 541.3 g in Gr. 2 (P = 0.9186) (Table 2), and we did not find a statistical difference between groups.
| Gr. 1 – 68 | Gr. 2 – 51 | P (Gr. 1-2) | OR |
| Preeclampsia | 2 (2.9%) | 2 (3.9%) | 0.7773 | 1.33 |
| No. of deliveries vaginal | 67.6% | 70.6% | | |
| Cesarean section | 32.3% | 29.4% | 0.7651 | 1.0909 |
| Gestational weeks of delivery | 36-40 | 36-40 | | |
| Preterm delivery < 37 weeks | 3 (4.41%) | 3 (5.88%) | 0.7311 | 1.33 |
| Birth weight (g) | 3495 ± 493.5 | 3570 ± 541.3 | | |
Table 2: Clinical data of women with GDM.
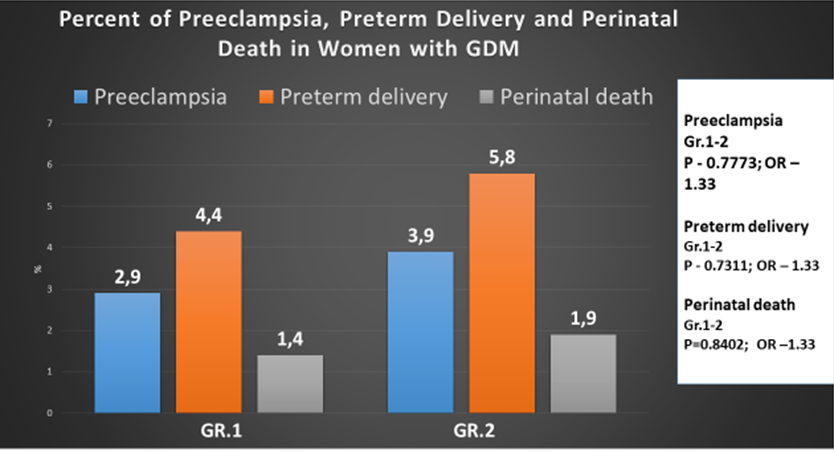 Figure 2: Percent of preeclampsia, preterm delivery, and perinatal death in women with GDM.
Figure 2: Percent of preeclampsia, preterm delivery, and perinatal death in women with GDM.
In both groups, the percentage of macrosomia was high, despite good glycemic control maintained through pregnancies: 20.0% and 23.0%, for Gr. 1 and Gr. 2, respectively (P = 0.9236, OR -1.0256), and again no statistical difference was found between the groups. Thus, we have found no relationship between the different treatment methods (insulin and metformin) used in patients with GDM and macrosomia development risk (Table 3).
| n | Gr. 1 – 68 | Gr. 2 – 51 | P (Gr. 1-2) | OR |
| Macrosomia | 14 (20.5%) | 12 (23.5%) | 0.8928 | 0.9630 |
| Neonatal hypoglycemia | 3 (4.41%) | 1 (1.9%) | 0.9236 | 1.0256 |
| Respiratory distress syndrome | 2 (2.94%) | 2 (3.92%) | 0.9694 | 0.9899 |
| Major congenital malformations | 1 (1.4%) | – | | |
| Stillbirths | 1 (1.4%) | – | | |
| Neonatal deaths | – | 1 (1.9%) | | |
| Perinatal mortality per 1000 births | 11.4 | 11.9 | | |
Table 3: Clinical data of women with GDM.
The percentage of neonatal hypoglycemia was lower in Gr. 2 (1.9%) than in Gr. 1 (4.41%) (P = 0.9236, OR – 1.0256), however, we did not find a statistical difference between the groups either (Table 3). The percentage of respiratory distress syndrome was 2.94% and 3.92% for Gr. 1 and Gr. 2, respectively (P = 0.9694, OR – 0.9893), with no statistical difference between the groups. Major congenital malformations were observed only in 1 newborn born to a mother from Gr. 1.
4. Discussion
Until recently, insulin therapy has been the most common treatment for patients with gestational diabetes [32]. Approximately 50% of women with GDM are prescribed insulin therapy for treating hyperglycemia [27, 33], but for the last few years, metformin has been increasingly used in the treatment of GDM [34]. In 2019, a meta-analysis was published that included 28 studies (3976 women with GDM), this study compared the weight of the newborn and different treatments for gestational diabetes: insulin and metformin. It was shown that newborns treated with metformin had a lower birth weight (mean difference – 107.7 g, 95% CI) -182.3 to -32.7), reduced risk of LGA (OR 0.78; 95% CI 0.62-0.99) and macrosomia (OR 0.59; 95% CI 0.46-0.7) than in newborns whose mothers received insulin [35]. In the Australian-New Zealand clinical trial, 751 women with GDM were enrolled to receive either metformin or insulin; researchers did not find significant differences in the results obtained, such as neonatal hypoglycemia, respiratory distress syndrome, hyperbilirubinemia, low Apgar scores, birth trauma, and preterm birth [27].
In 2017, a review was published (n = 1487) which included 8 randomized controlled trials evaluating the effects of oral antidiabetic pharmacological therapy (metformin, glyburide, and acarbose) in the treatment of pregnant women with GDM, the results showed that the benefits and potential harms of these treatments compared with each other are unclear [36]. Other meta-analyses have compared glibenclamide or metformin with insulin in women with gestational diabetes requiring medical treatment. The following conclusions were made: glibenclamide is clearly inferior to both insulin and metformin, while metformin (plus insulin if needed) works slightly better than insulin. Less frequent episodes of gestational hypertension have been observed in patients taking metformin compared with glibenclamide or insulin [6, 37], but the use of metformin has been associated with an increased risk of preterm birth compared with insulin [37].
Metformin reduces gluconeogenesis and reduces insulin resistance at the receptor level. It practically cannot cause episodes of hypoglycemia and also does not lead to weight gain. The results of the use of metformin during pregnancy in patients with polycystic ovary syndrome are well known. Therefore, it can be considered that metformin may be an alternative to insulin in the treatment of gestational diabetes. Another multicenter, open-label, randomized, parallel-group clinical trial conducted in 2 hospitals in Malaga, Spain, evaluated the efficacy of metformin vs. insulin in 200 women with GDM and concluded that metformin treatment was associated with better postprandial glycemic control than insulin, lower risk of hypoglycemic episodes, less maternal weight gain, most perinatal outcomes were similar across groups [18].
The primary objective of our study was to evaluate glycemic control in women with GDM treated with metformin or insulin. After the introduction of insulin and metformin, HbA1c levels statistically decreased in both groups: Gr. 1 – 6.01 (0.5) and Gr. 2 – 5.9 (0.6), and before delivery, we did not find a statistical difference between groups (Р = 0.3239). The results of our studies show that insulin, like metformin, effectively controlled glycemia during pregnancy in patients with GDM. In addition, from the start of the treatment before delivery, women from Gr. 2 (metformin-treated group) gained less weight compared to women from Gr. 1 (insulin-treated group) (1.89 ± 3.88 vs. 4.53 ± 3.67 kg; P = 0.003). So, maternal weight gain was significantly lower in the metformin group. However, other studies have shown different results from ours, as a randomized prospective study published in 2021 found no trend toward lower weight gain during pregnancy in women in the metformin compared to the insulin group [18].
The secondary objective of this study was to assess the incidence of preeclampsia, preterm delivery, cesarean section, macrosomia, malformations, respiratory distress syndrome, and perinatal mortality. The incidence of preeclampsia in the insulin-treated group was lower than that in the metformin-treated one, and the difference was not statistically significant (P = 0.7773, OR – 1.33). The percentage of preterm deliveries was lower in Gr. 1 (insulin-treated group) compared to Gr. 2 (metformin-treated group), though there was no statistical difference between the groups (P = 0.7311, OR – 1.33). In our study, perinatal mortality was observed in both groups, it comprised 1.4% in insulin-treated patients and 1.9% in metformin groups, and no statistical difference was found between the groups (P = 0.8402, OR -1.33). Stillbirth was registered only in Gr. 1, and its rate was 1.4%, while neonatal death was only in Gr. 2 (1.9%). The percentage of cesarean section was high in both groups: in insulin-treated patients, it was 32.3%, and in metformin-treated patients, it was 29.4%, and no statistical difference was found between the groups (P = 0.7651, OR – 1.0909). In both groups, the percentage of macrosomia was high, despite good glycemic control maintained through pregnancies, 20.0%, and 23.0%, for insulin and metformin-treated patients, respectively, and no statistical difference was found between the groups.
We did not find significant differences between the groups (insulin and metformin) in perinatal outcomes. Our study shows that the use of metformin in the treatment of patients with gestational diabetes is effective and safe. 119 women to receive either metformin or insulin therapy, finding no significant difference in the incidence of preeclampsia, preterm delivery, cesarean section, macrosomia, malformations, respiratory distress syndrome, and perinatal mortality. While comparing insulin and metformin therapy, we observed that metformin and insulin are equally effective in the management of gestational diabetes and that metformin was linked to less maternal weight gain (p = 0.003).
5. Summary
There was no significant difference in the efficacy of metformin and insulin in controlling diabetes in pregnant patients from the two groups, therefore, the efficacy of metformin and insulin was found to be comparable in the management of pregnancy with diabetes in this study. The percent of preeclampsia, preterm delivery, macrosomia, neonatal hypoglycemia, respiratory distress syndrome, and perinatal death was similar in both groups. We observed that only maternal weight gain was lower in the metformin group.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Omori Y, Jovanovic L. Proposal for the reconsideration of the definition of gestational diabetes. Diabetes Care. 2005;28(10):2592-593.
- International Diabetes Federation. IDF Diabetes Atlas 9th ed. 2019.
- Hartling L, Dryden DM, Guthrie A, et al. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep). 2012;(210):1-327.
- American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35 Suppl 1(Suppl 1):S11-63.
- Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341-63.
- Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102.
- Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-82.
- Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131 Suppl 3:S173-211.
- Reader D, Splett P, Gunderson EP, et al. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dietitians on pregnancy outcomes. J Am Diet Assoc. 2006;106(9):1426-33.
- Institute of Medicine (US) Food and Nutrition Board. Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. Washington (DC): National Academies Press (US); 1998.
- Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, et al. Physical Activity Programs during Pregnancy Are Effective for the Control of Gestational Diabetes Mellitus. Int J Environ Res Public Health. 2020;17(17):6151.
- Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30 Suppl 2:S251-60.
- ElSayed NA, Aleppo G, Aroda VR, et al. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S254-S266.
- Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus. Endocr Pract. 2021;27(6):505-37.
- Pertot T, Molyneaux L, Tan K, et al. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care. 2011;34(10):2214-216.
- Mukherjee SM, Dawson A. Diabetes: how to manage gestational diabetes mellitus. Drugs Context. 2022;11:2021-9-12.
- Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57(3):308-14.
- Picón-César MJ, Molina-Vega M, Suárez-Arana M, et al. Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: randomized prospective trial. Am J Obstet Gynecol. 2021;225(5):517.e1-517.e17.
- Bao LX, Shi WT, Han YX. Metformin versus insulin for gestational diabetes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34(16):2741-753.
- HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
- Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780-86.
- Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113(1):12-6.
- Cosson E, Cussac-Pillegand C, Benbara A, et al. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: A retrospective observational study. Diabetes Metab. 2016;42(1):38-46.
- Conde-Agudelo A, Belizán JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107(1):75-83.
- Ovesen PG, Jensen DM, Damm P, et al. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. a nation-wide study. J Matern Fetal Neonatal Med. 2015;28(14):1720-724.
- Hildén K, Hanson U, Persson M, et al. Overweight and obesity: a remaining problem in women treated for severe gestational diabetes. Diabet Med. 2016;33(8):1045-051.
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003-015.
- Tertti K, Ekblad U, Vahlberg T, et al. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud. 2008;5(2):95-101.
- Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373(9677):1789-797.
- Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636-44.
- Rosenstein MG, Cheng YW, Snowden JM, et al. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. 2012;206(4):309.e1-7.
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21 Suppl 2:B161-7.
- Sweeting A, Wong J, Murphy HR, et al. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43(5):763-93.
- Lindsay RS, Loeken MR. Metformin use in pregnancy: promises and uncertainties. Diabetologia. 2017;60(9):1612-619.
- Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002848.
- Brown J, Martis R, Hughes B, et al. Oral anti-diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;1(1):CD011967.
- Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta-analysis. PLoS One. 2014;9(10):e109985.
![]() and Shelestova E
and Shelestova E

