The tapeworm Echinococcus granulosus causes a destructive zoonotic disease named cystic echinococcosis (CE), a crucial global issue for the public health sector. Nowadays, human hydatid disease is a worldwide challenge, and it is considered a recurrent disease in locations where it was formerly at low rates. Significant attempts and studies have been put into controlling and preventing the transmission of E. granulosus from dogs to humans, but the outcome is not considerable. An outstanding trait of the intermediate host-parasite relation is the host’s protective immunity against infection by the oncosphere phase of taeniid cestodes. Due to this noble trait, we can develop different approaches to control hydatid disease through livestock vaccination. Moreover, the cestode isolates are barely distinguished, and due to a lack of knowledge about reaction mechanisms, their effect on innate immunity is not entirely tested. Yet, issues related to these topics regarding the purification of immunostimulatory molecules, their side effects, and their action on parasites remain struggles that need to be discussed. This review discusses multiple facts challenging autoimmune and immune responses guarding E. granulosus against suppression to reduce intense host damage.
Despite numerous studies and controlling actions, echinococcosis is still a disease of global importance [1, 2]. In some parts of the world, cystic echinococcosis (CE) is a reappearing disease in locations formerly at low rates [3, 4]. The adult form of Echinococcus granulosus sensu lato is located in the definitive host’s small intestine [5]. Gravid proglottids pass eggs into feces, and then a proper intermediate host ingests them [6]. The eggs hatch in the small intestine and release six hooked oncospheres that can pass through the intestinal wall and use the circulatory system to roam into different organs, particularly the liver and lungs [7, 8]. A thick-walled hydatid cyst forms from the development of the oncosphere. Progressive growth of hydatid cysts results in the production of daughter cysts and protoscolices [9]. The infection transmits to the definite host by ingesting cyst-infected organs of the intermediate host [10]. Next comes the evagination of protoscolices. Evaginated protoscolices connect to the intestinal mucosa and develop into adult phases [11]. E. granulosus employs the hydatid cyst structure exposed to the host and the interior portions of the cyst to elude host immune responses. Cyst comprises a fibrous adventitial layer produced from the host inflammatory responses [12]. CE includes two layers: an inner cellular germinal layer (GL) and an outer acellular carbohydrate-rich laminated layer (LL) [13]. GL and LL are critical elements in arousing innate immune responses in the host-parasite relationships because of the accumulation of diverse influential antigens and molecules [14–16]. The acellular LL is a carbohydrate-protein structure; the polysaccharide part’s main constituents are galactose, galactosamine, and glucosamine [17]. A perinuclear layer and a distal cytoplasmic syncytium are two components of the GL [18]. The perinuclear consists of flame, muscle, tegumental, glycogen-storing, and undifferentiated cells that involve lysosomal-like bodies [19]. Hydatid cyst fluid (HCF), which flows inside the cyst, collects a diverse range of products discharged by the cellular, GL of the cyst wall and protoscoleces [20]. Moreover, hydatid fluid collects different plasma proteins of the host (mainly immunoglobulins and albumin) that bypass the cyst wall in unknown processes [21, 22]. The distinct feature of E. granulosus metacestode infections is that the gradually growing hydatid cysts might not get diagnosed for months or even years after the primitive disease has happened [23]. Immunologists are interested in these cysts because of their perseverance [24]. Once these cysts are formed, seemingly host immune responses cannot affect them [25]. To envision the various immune stimuli affecting the host and to describe diagnostic and therapeutic tools, it is vital to have a decent comprehension of biological events happening throughout the infection.
E. granulosus can use passive escape and immunomodulation as mechanisms that help overturn the host’s immune responses [26]. Passive escape through which the parasite turns into a hydatid cyst to avoid the harm caused by the immune system and immunomodulation, the parasite purposefully cooperates with the host immune system to lower the effect of the immune responses [27, 28]. Based on the conceptions, the immune response against the parasite has been categorized into two stages: pre- and post-encystment [29, 30]. The differentiating factor between the two stages is the genesis of the LL around the growing infective oncosphere. The parasite must bypass chemical and physical barriers before reaching the internal part of the body. Following the initial infection, protoscolex induces a robust inflammatory response. The inflammatory response recruits eosinophils, lymphocytes, macrophages, and neutrophils, leading to complement activation.
Physical and chemical barriers
Epithelia contain different parts like the skin, the linings of the body’s tubular structures, and the respiratory, gastrointestinal, and urogenital tracts [31]. Epithelia provides an expanded surface for optimized absorption and enzymatic digestion of complex nutrients. The glycocalyx atop the epithelial cells can stop diffused microorganisms in the mucus layer from binding to the epithelial cells [32]. The structure of this barrier is created by polymeric, gel-forming mucin. Goblet cells produce, preserve and secret the huge O-linked glycoproteins and other protective factors [33]. Immune regulation of goblet cells during infection affects the generation and attributes of mucin and, finally, the function of the barrier [34]. Mucin’s role as a protective barrier of the host is well accepted as a vital feature of innate defense [35]. On top of creating a physical barrier, the disulfide-linked mucin polymers operate as lubricants, avert dehydration of the epithelial surface and provide distinct ligands to bind pathogens [36]. The impact of acid pepsin on the rupture of the shells or activation of the free oncospheres didn’t confirm, while NaHCO3 and NH4OH ruptured shells without activation of oncospheres [37, 38]. It is good to mention that pancreatin and trypsin did activate some of the free oncospheres. Pancreatin and trypsin activating effects can be amplified by factors like bile, salts, and cholesterol [39]. The impact of whole bile is more distinct and shows more synergy than individual bile salts tested. Alongside immunological mechanisms, other factors like bile, intestinal motility, gastric juice, secretions of the pancreas, and intestinal microflora form a crucial defense line preventing microorganisms’ invasion of the gut [40]. Unfortunately, there is not enough reliable data on the effect of the gastric acidic barrier on the Echinococcus granulosus oncosphere. Paneth cells create antibacterial and antifungal peptides named cryptdins or α-defensins down the intestinal tract that are located in the base of the crypts in the small intestine under the epithelial stem cells [41, 42] (Figure 1). Despite a vast range of studies on host-relationship in human CE during the last decade, determining the whole mechanisms that result in the disease demands more effort. We need to describe the processes that manipulate the host immune response to guard the E. granulosus against termination. Based on recent experimental studies, the parasites are more than just evading the immune responses actively. They can manipulate the hormonal microenvironment within the host to favor their residency and growth [43]. The parasites’ gains from hormonal exploitation are high enough to develop structures similar to steroid and protein hormone receptors differentiated in vertebrates [44]. These structures can bind to hormonal metabolites produced by the host [45]. Becoming aware of the mechanisms in which the host endocrine system aids the growth and development of a parasite, alongside analyzing the parasite hormone receptors that are engaged in the process, can help the creation of hormonal analogs and drugs that can uniquely impact the parasite.
 Figure 1: Physical and chemical and biochemical barriers
Figure 1: Physical and chemical and biochemical barriers
Neutrophils
The first detective and eliminative agents against parasites are neutrophils, but they have a weakness that allows parasites’ metabolites to intrude with their biological activities [46]. Neutrophils contain granules known as lysosomes with peptides, enzymes, and proteins that can initiate an intracellular antiparasite response [47]. Neutrophils kill the surrounding microorganism by secreting other toxic products [48]. The most critical poisonous products created by neutrophils are nitric oxide (NO), hydrogen peroxide (H2O2), and directly toxic superoxide anions. These toxic products originate from lysosomal NADPH oxidase [49]. Neutrophil elastase (NE) is a protease exuded by activated neutrophils with the ability to digest parasites’ bodies and cause neutrophil chemotaxis. The oncospheres of E. granulosus contain EgKI-1, a potent NE inhibitor of the secretory type that can guard this stage against the host immune responses [50, 51]. Suppose the hydatid cyst ruptures during the chronic stages of echinococcosis, in that case, neutrophils become attracted to kill the protoscoleces, which can cause high antigen B (AgB) in hydatid cyst fluid [52]. AgB can crucially lower neutrophil recruitments as a potent protease inhibitor [53]. This delays the killing of protoscoleces by neutrophils and provides time for the larvae to develop into larger cysts leading to secondary echinococcosis [54]. In both chronic and acute stages of the disease, E. granulosus survival highly depends on preventing neutrophil chemotaxis and NE secretion [55].
Macrophages
E. granulosus larvae primarily reside in the liver, resulting in CE, a tumor-like parasitic disease. CE is distinguished by enhanced infiltration of different immune cells, like macrophages around the lesion, forming an immunosuppressive microenvironment [54, 56]. The immunosuppressive microenvironment mediates the maintenance of the infection [57]. Despite many studies on this matter, the function of hepatic macrophages engaged in the host defense against E. granulosus infection stays inadequately defined [58]. A considerable part of CD68+ macrophages piled up around the metacestode lesion in the liver of human CE samples alongside M1 phenotype (pro-inflammatory) and M2 phenotype (anti-inflammatory) are significantly higher in close liver tissue (CLT) in comparison to distant liver tissue (DLT) [59]. Also, the M2 phenotype forms the general population of macrophages. Moreover, mice infected by E. multilocularis represented a massive increase in macrophage infiltration in the liver during the first five days. The infiltrated macrophages were mostly monocyte-oriented (CD11bhi F4/80int MoMFs) that selectively developed to the M1 phenotype (iNOS+) at the primary phases of E. multilocularis infection [60]. Differentiated M1 phenotype macrophages turn into anti-inflammatory macrophages of the M2 phenotype (CD206+) during the chronic phase of the disease. Hepatic macrophages represent a dual function in interaction with E. multilocularis metacestodes [61]. M1 phenotype macrophages do the early larvae clearance, while M2 phenotype macrophages favor the continuous metacestode infection [62]. Macrophages can also activate T cells by providing antigens [63]. Macrophages release cytokines that contribute significantly to local inflammation and other nonadaptive compelling responses during the first days of a new infection [64]. Activated macrophages produce NO, essential to preventing the diffusion of the hydatid cyst layer. NO (nitric oxide) synthase response can threaten E. granulosus survival, but the LL guards it by upregulating the host arginase pathways [65]. It has been mentioned that IL-12 has a regulatory function in innate immunity in the intermediate hosts against CE infection. The Echinococcus species operate several approaches during the infection period to locate peacefully in their respective host without stimulating destructive immune responses like releasing reactive inflammatory materials [66].
During the infection period, Echinococcus species managed to enhance several approaches to locate unharmed within their respective host without stimulating destructive immune responses like releasing reactive inflammatory materials [67]. The miRNAs are a group of tiny regulatory non-coding RNAs that favor the initiation of host-pathogen cross-talk within the infection [68]. The miR-71 is highly protected in helminths, and it has lately been discovered in nematode exosomes, the same as in the sera and fluids of infected mice and humans [69]. It has been lately described that miRNAs can function as molecules that permit modulation of the host’s innate immunity [70]. Diffusion of parasite-derived miR-71 into hosts might impact the practices of macrophages [71]. Although the function of miR-71 during the infection period is inadequately determined [72].
Dendritic cell
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) and the only APC able to activate naive T cells [73]. Migration of immature DCs begins from the blood and ends by locating in the tissues. DCs can be macropinocytosis and phagocytic, engulfing a vast amount of the surrounding extracellular fluid [74]. Once DCs face a pathogen, they immediately mature and move to the lymph nodes [75]. DCs develop several receptors, including C-type lectins, mannose receptors (MR), and TLRs, with the potential to engage with Echinococcus and its products [76]. Echinococcus spp. during larval phases can benefit excretory-secretory products (ES) to adjust the function of DCs [77]. It has been reported that hydatid cyst fluids and antigen B of E. granulosus can modify DC differentiation and cytokine secretion. Also, the LL can cause the irregular maturation of DCs [78]. Based on our data, we can claim that Echinococcus spp. (ES) has a broad suppressive impact on DC activation and cytokine cascade that modifies the extent of Th1 responses. Therefore, we suppose that inhibition of DC maturation and function results in a possible immunosuppressive mechanism caused by E. granulosus in the same way as Schistosoma japonicum, Heligmosomoides polygyrus, and some other pathogens [79, 80]. A recent study showed that lipopolysaccharides found in E. granulosus hydatid fluid intervene in monocyte precursor differentiation into immature DCs and prevent their maturation process [81].
Mast cell
In cestode infections, mast cells are significant effector cells even though their necessity for nematode clearance seems to differ with the species of cestode [82, 83]. Mast cells have lots of granules containing heparin, proteases, and histamine that can exude cytokines such as IL-4 and IL-5 besides leukotrienes and chemokines in activation [84]. Standard activation causes degranulation of mast cells, leading to the binding of immunoglobulin (IG) E to and cross-linking of the FcεR [85]. Resident eosinophils can be activated by the histamine secreted from mast cells.
Eosinophils
Aside from mast cells, eosinophils are the most normal cells with the ability to permeate the zone of cestode infection. Eosinophils have granules containing several cationic proteins that can secrete a group of pro-inflammatory cytokines, lipid mediators, and chemokines, making them essential effector cells [86]. The number of peripheral blood eosinophils increases significantly during parasitic infections. This happens due to Th2 cell-derived IL-5, IL-3, and GM-CSF [87]. Eosinophils are recruited by eotaxin, the selective eosinophil chemokine, from blood circulation to inflamed or harmed tissues. The recruited eosinophils get prepared by interaction with connective tissue matrix proteins like laminin and fibronectin before becoming activated by cytokines via receptor-mediated signals [88]. Afterward, the activated eosinophils release helminthotoxic or histotoxic reactive oxygen species (ROS) and granular proteins [89]. A diverse range of cell surface receptors is present on eosinophils for improved cell signaling associated with apoptosis, adhesion, chemotaxis, degranulation, production of cytokines and chemokines, respiratory burst, and survival. All of these mentioned can be tightly related to eosinophil-mediated tissue inflammatory responses in helminth infection. The latest experimental studies have indicated that eosinophils can act as APCs [90]. Eosinophils have the capability of providing and presenting a diverse range of parasitic, microbial, and viral antigens. Even though eosinophils are engaged in tissue inflammatory responses in helminth infections, their protective function against tissue-invasive helminths stays disputable [91]. Bilobed nuclei and four primary granules can distinguish eosinophils. The primitive granule is the central creation zone of the Charcot-Leyden crystal protein (CLC or galectin-10) [92]. There is a probability that CLC is engaged in the interactions between eosinophils and the numerous carbohydrate remainders that parasitic worms convey on their surfaces [93]. Cytotoxic granular proteins contain eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPO), eosinophil cationic protein (ECP), and significant essential protein (MBP) that all of which are located in the crystalloid secondary granule beside several cytokines [94]. Eosinophils move to the peripheral blood circulation and migrate to particular tissues, mainly the gastrointestinal tract, with the help of eotaxin-1, adhesion molecules, and IL-5. ROS are toxic compounds secreted by eosinophils alongside other toxic granule proteins like EDN, MBP, and ECP [95, 96]. ROS are produced by the NOX family of NADPH oxidase and can be triggered by IL-3, IL-5, PAF, C5a, GM-CSF, PMA, and eotaxin [97].
Natural killers
Natural killers operate in immediate reaction to harmful cells and do not need to be previously activated. Because of this characteristic, they are considered cytotoxic lymphocytes, highly important to the innate immune system [98]. Natural killers have two noble features that amplify their importance and value. The first is their distinct capability to identify stressed cells without demanding prior activation. This results in a much faster immune response [99]. The second one is their determined function in tumor surveillance, represented in humans and mice [100]. Even though natural killers are famous for their advantageous effect on innate immune responses against intracellular microorganisms involving: protozoa, viruses, and bacteria, their role in Echinococcus infection has not been investigated through a decent number of studies. It has indicated that those with active CE cysts have relatively more natural killer cells (CD56+CD8-) in their PBMC [101] (Figure 2).
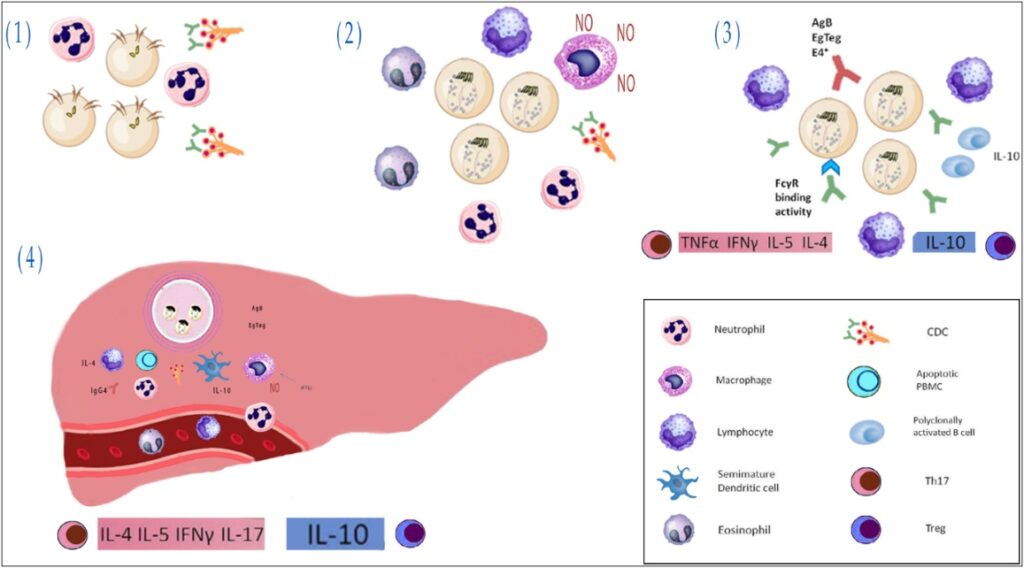 Figure 2: Innate immune response in intestinal Echinococcus granulosus sensu lato infection.
Figure 2: Innate immune response in intestinal Echinococcus granulosus sensu lato infection.
Complement system
We consider the complement system a critical effector in the innate immunity system. The complement system can directly intervene in the suppression of pathogens through cell lysis or stimulation of inflammation or opsonization indirectly [102]. It consists of a cell surface and plasma proteins practically linked by a cascade activation mode [103]. It has been reported that the character of many parasitic helminths involving E. granulosus can initiate the complementary pathway of the complement system [104]. Even though the complement can lyse protoscoleces of E. granulosus, the parasite can consume the supplement with the help of some exudative products [105]. This potency has been represented as the foundation of an invasion mechanism by the parasite. Due to the remarkable rise in the rate of C3 in patients struggling with hydatid disease, there might be the possibility of local consumption that results in systemic consumption in cysts with more activity [106]. As the LL is penetrable to host macromolecules in the established cyst, the outer syncytial tegument of the GL is always revealed to complement levels possibly not varied from the ones in intracellular fluid [107].
Toll-like receptors
Identifying parasite components is significant for the operation of a proper innate response. Such identification is known to happen across the interaction with pattern recognition receptors like toll-like receptors (TLRs) [108]. Lately, it has been indicated that TLRs are crucial for activating immune cells, involving DCs and macrophages through identifying microbial and parasitic components [109]. TLRs are vital in antigen recognition and are primarily discovered as pattern recognition receptors [110]. It is indicated that helminthic products, like E. granulosus antigen B, prevent the activation of DCs as a response to conventional TLR ligands such as lipopolysaccharide [111]. Nonetheless, studies have indicated that TLR2 and TLR4 have a significant function in recognizing helminthic products by macrophages and DCs and expanding Th2 responses [112]. The raised expression of IL-23 and TLR2 can have a crucial role in adjusting the infiltrative tissue growth of the parasite and its resistance in the human host [113]. Distinguishing the real impact and mechanism of TLRs and related cytokines in immune tolerance and the development of echinococcosis in humans and animals demands a bigger population scale and continuing attempts [114]. Based on E. granulosus as an extracellular helminthic parasite, TLRs expressed on the cell surface can be involved in recognizing surface hydatid antigens. It has been suggested that TLR2 and TLR4 are the best among TLRs in recognizing surface hydatid antigens [115]. Moreover, there is not much information about the function of the recently discovered cytokine, IL-17. Reviews indicate that TLR2 and TLR4 additionally function in E. granulosus and host interactions [116]. Due to their high expression in patients with chronic CE, it can be concluded that their involvement is not limited to the first step of immune recognition, and they remain a part of the long-term retention of immune tolerance against an established hydatid cyst [117] (Figure 3).
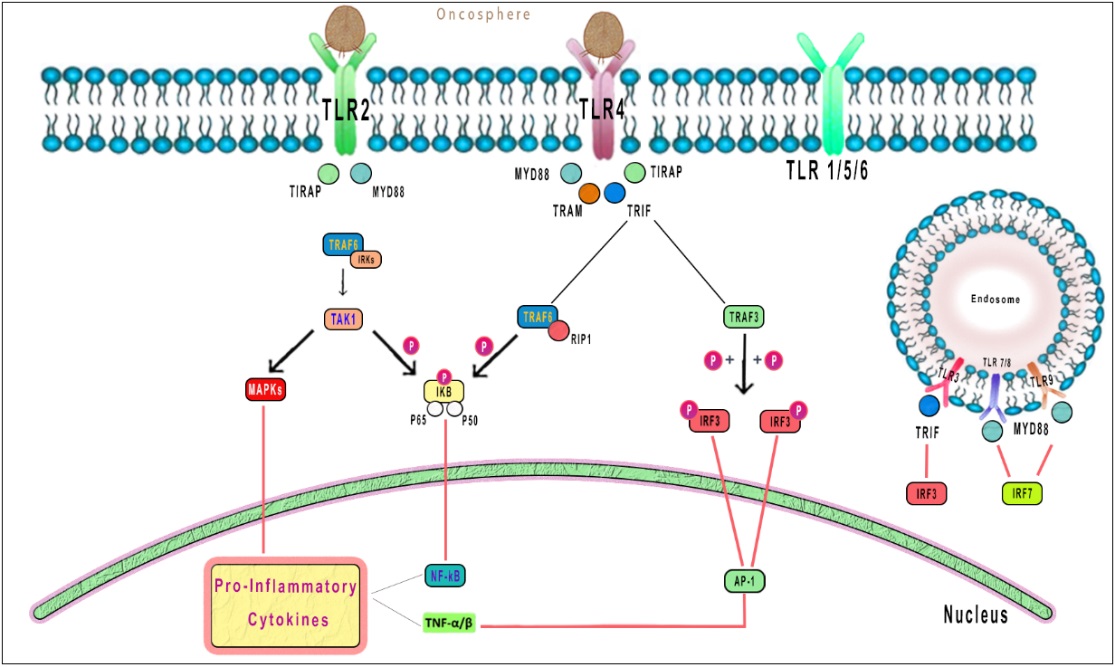 Figure 3: TLR2 and TLR4 additionally function in E. granulosus and host interactions. Due to their high expression in patients with chronic CE, it can be concluded that their involvement is not limited to the first step of immune recognition and they remain a part of the long-term retention of immune tolerance against an established hydatid cyst.
Figure 3: TLR2 and TLR4 additionally function in E. granulosus and host interactions. Due to their high expression in patients with chronic CE, it can be concluded that their involvement is not limited to the first step of immune recognition and they remain a part of the long-term retention of immune tolerance against an established hydatid cyst.
Due to the secretion of hydatid cysts, many immunomodulatory molecules get exposed to the host’s immune system. Researchers are searching for new approaches to enhance disease control and fully comprehend parasite biology. They have discovered and differentiated several E. granulosus antigenic molecules involving: EG95 [118], EgA31 [119], elongation factor b/d [120], EPC1 [121], EgTeg [122], cyclophilin [123], HSP70 [124], TPx [125], antigen 5 and antigen B [126, 127].
Developing vaccines against CE as an essential zoonotic parasite infection is an interesting approach to controlling this disease. Since CE infects humans, wildlife, and livestock worldwide, the vaccine against the larval and adult phases of the Echinococcus granulosus life cycle is the primary step to providing broad protection against it [128]. Vaccines trigger hosts’ immune systems as the body’s direct defending approach against infections. Different strategies can be used to find an antigen with a high level of immunogenicity, such as immune-informatics, immune-genomic and proteomic, system vaccinology, and bioinformatics modeling [129]. Recently, the next generation of vaccine is emerged by applying new technologies and combing different scientific fields [130]. The main challenges nowadays for developing a vaccine against this multi-phase parasite result from funding sources and issues related to policy and culture [131]. Accordingly, finding a multivalent vaccine that functions against different phases of the E. granulosus life cycle is a cost and time-effective strategy [132]. Developing vaccines is a multidisciplinary field and applies host-pathogen interaction studies, immunological data on MHC molecule’s function, immune signaling pathways, and bioinformatics [133]. Several antigens are used to generate recombinant vaccines for E. granulosus based on epitopes. To design these vaccines, in silico immunoinformatics analysis is commonly applied [134]. Some types of in silico research focus on the B-cell epitopes prediction to introduce antigen candidates without considering T-cell epitopes [135, 136].
EG95: The EG95 vaccine is a 16.5 KD protein expressed from oncosphere parasite mRNA in fusion with glutathione S-transferase to produce a fusion recombinant protein-based vaccine [137]. There is restricted information about the cellular response of recombinant vaccines for E. granulosus [138]. Immunization of mice bearing E. granulosus hydatid cyst with BCG attached EG29 vaccine has reduced the cyst volume by about 93% [139]. This observation is related to the increased level of IFN, IL-2, and TNF and reduced level of IL-4, which proposes this vaccine function through triggering the Th1 response. Since many host protective antigens of the oncosphere are considered to develop vaccines against taeniid cestode parasites, comparing the EG95 effectiveness on these antigens is now required [140].
Although researchers provided several reports on CE as one of the significant pathogenic infections due to its multi-host infection property worldwide, determining the disease’s detailed cause requires more investigations. Moreover, researchers should study the parameters in the host immune system that reduce severe immune responses and protect E. granulosus from removal. We know that the innate response against pathogens prepares a static barrier and plays a vital role as the critical director of the adaptive immune response, which finally removes parasites from the body. Introduced vaccines for this disease also induce a similar immune response. However, some mechanisms provide resistance and neutralize immune responses. There are some areas regarding this challenge that require more investigations which are mentioned below:
- The effect of recombinant proteins of the Eg-29 family on innate immune response, considering complements.
- The hydatid’s external secretion affects dendritic and other immune cells.
- The effect of EgAg5 and EgAgB on dendritic and other immune cells.
- The impact of inhibitors belonging to the Kunitz family on adaptive and innate immunity activation.
- Molecular mechanism of conditioning of DCs and macrophages with LL particles.
- The results of LL mucins interaction with liver macrophages.
Furthermore, it demonstrated that the thin coated layer on the surface of the cestode cuticle that is rich in carbohydrate moieties in collaboration with secreting molecules provides the first contact with the host’s innate immune system. Therefore, the innate host recognition system chooses the pattern recognition receptor since they detect damaged tissues and carbohydrates that do not belong to the host. Up to now, different therapies and protection approaches have been introduced, such as vaccines, chemotherapeutic drugs, and new therapeutics. However, there are still several issues that need to be addressed. For example, recombinant vaccines have been produced to immunize sheep against CE by interfering with their life cycle. This indirectly prevents the spreading of the infection from dog to human, considering the possibility of using the same place by sheep and dogs. However, vaccination of dogs provides direct and cost-effective protection against CE since dogs are determined to host CE and the number of dogs in the area is mostly fewer than sheep. Chemotherapeutic drugs also face challenges, although they improved treating the cestodes in clinics. For example, we can imply the lack of a specific delivery site for the drug, which provides several side effects. Furthermore, it is required to design siRNA and inhibitors against the TLRs and anti-apoptotic proteins by applying in silicon approach to investigate whether this strategy will improve the treatment level of CE. It is also discussed that studying resistance genes and vaccines in microbial infections will provide helpful information to clarify the function of the innate immune system in resistance to an infectious organism.
![]() 1, Charbgoo A
1, Charbgoo A![]() 1, Borji H
1, Borji H![]() *2 and Hajjafari A
*2 and Hajjafari A![]() 3
3


