Abstract
Ganoderma lucidum (L) is considered an effective medicinal mushroom. The main goal of this research was to investigate whether the beneficial effects of G. lucidum found in various in vitro or animal studies can be translated to chronic metabolic diseases such as diabetes in humans. Here, we present a case study of a person with diabetes after treatment with G. lucidum and provide important information on fasting blood glucose (FG) levels, glycated hemoglobin (HbA1c), lipid profile, and various other blood parameters. After taking G. lucidum hot water extract for three months, FG levels decreased from 198 mg/dL to 177.3 mg/dL. HbA1c fell by 1.5%. Serum total cholesterol (TC) decreased from 210 to 170 mg/dL, and triglyceride (TG) levels decreased from 220 to 150 mg/dL, while serum high-density cholesterol (HDL-C) increased from 25 to 35 mg/dL. Low-density cholesterol (LDL-C) only decreased by 9 mg/dL. Serum creatinine, alkaline phosphatase (ALP), alanine aminotransferase (ALT), and total bilirubin levels were not changed. Therefore, it is concluded that G. lucidum hot water extract had antidiabetic and antidyslipidemic effects without affecting hepatorenal functions.
Keywords
Ganoderma lucidum, diabetes, glycemia, Ganotea, hypercholesterolemia, hypertriglyceridemia, dyslipidemia
Abbreviations
FG: fasting blood glucose, ALP: alkaline phosphatase, ALT: alanine aminotransferase, OSCC: oral squamous cell carcinoma, BMI: body mass index, CBC: complete blood count, Hb: hemoglobin, HbA1c: glycated hemoglobin, TG: triglyceride, TC: total cholesterol, HDL-C: high-density cholesterol, LDL-C: low-density cholesterol
1. Introduction
Diabetes represents a major public health burden worldwide, including in Bangladesh, where the prevalence of this disease is increasing every year [1]. All over the world, in addition to allopathic, alternative treatments are being considered to combat diabetes and its associated costs. This article examines a case report demonstrating an alternative treatment approach for diabetes using the G. lucidum mushroom. Due to its medicinal benefits, G. lucidum (L) has been the subject of hundreds of investigations worldwide in numerous areas, from cancer to inflammatory diseases, from metabolic to microbial diseases, but most of these have been in vitro or animal studies [2–4]. This fungus is relatively new to Bangladesh and is said to have many beneficial effects on human diseases. Accordingly, G. lucidum (L) is capturing a lucrative share of the herbal medicine market in Bangladesh. However, many contemporary doctors, not only in Bangladesh but also in Western and European countries, believe that the claims made in the commercials about the potential effectiveness of G. lucidum products are fake or untrue. These Western medicine physicians even claim that the effect of G. lucidum is paradoxical or due to the placebo effect. This doubt is probably due to at least two facts: i) the lack of adequate clinical trials outside China, the country of origin of G. lucidum, where it has been found and used to treat various diseases for more than two millennia [5–7] and ii) the effectiveness of G. lucidum has remained largely untested and unmonitored in areas outside China. The insufficient data on side effects, especially in Bangladesh, also contributes to physicians doubting the effectiveness of G. lucidum.
We previously reported that G. lucidum ethanol extract has potential utility in the palliative form of treatment of patients with oral squamous cell carcinoma (OSCC) [8]. Before reporting this effect in OSCC patients, we also previously reported that the ethanol extract of this mushroom protected against paracetamol-induced hepatotoxicity [9] and heavy metal-lead (Pb)-induced hemolytic toxicity [10] in rats and memory impairment in the amyloid beta peptide (Aβ1-42)-infused Alzheimer’s disease model rats [11, 12]. These results suggest that the beneficial effects of G. lucidum found in animal studies may potentially lead to beneficial effects in human diseases [8]. G. lucidum has also been shown to have antidiabetic effects in floxacin/streptozotocin-induced diabetes model animals [13–15], meaning that this fungus may have significant effects on diabetes in humans.
Diabetes is a serious health problem worldwide. Currently, 537 million adults aged 20 to 79 years are living with diabetes [16]. It is worth noting that many persons with diabetes would prefer to stop Western medication use. Others may be willing to switch from Western anti-diabetic medications to more natural remedies such as supplements. This is why we conducted this research. Like physicians, many consumers in Western countries are skeptical of fungal supplements that may cause side effects such as kidney failure and liver damage that are dangerous for consumers. This is partly because herbal and dietary supplements are not regulated like prescription medications in the United States, and there have been numerous reports of supporting concerns for contamination and fraudulent ingredients. However, the Eastern countries that have developed herbal medicines generally claim that they were designed and manufactured to maintain health and emphasize that they are naturally harmless because they contain no harmful chemicals. To eliminate this doubt, in this study, we did not use organic solvents such as ethanol or methanol to prepare G. lucidum extract, which may contain harmful chemical residues. Instead, G. lucidum powder, like tea prepared in water, was boiled in water for 30 minutes to make G. lucidum tea and taken twice a day for three months. Hereafter, the term ‘hot water extract from G. lucidum’ will be used synonymously as ‘Ganotea’ throughout the text, as it is now gradually becoming known by this name in Bangladesh. In contrast to the clear tea drink, the Ganotea appeared cloudy due to the insoluble particles and compounds in the woody pulp of G. lucidum. The extract is not filtered to remove its granular components. The extract had a bitter taste.
2. Case Presentation
2.1 Case report
A 54-year-old man had no family history of diabetes, obesity, or cardiovascular disease on either his mother’s or paternal side. He experienced exhausting fatigue and suffered from excessive thirst and hunger. He also had to urinate more frequently, although the amount of water he normally drank did not change significantly. Then he called the doctor. The doctor advised him to have a blood test done first. He was then referred to a diagnostic clinic and asked for a routine health check-up. His systolic blood pressure was 115–122 mmHg, and his diastolic blood pressure was 76–82 mmHg. His body weight was 156.528 pounds, his height was 5 feet and 4 inches, and his body mass index (BMI) was 26.9. His laboratory tests included a complete blood count (CBC), fasting blood glucose (FG), hemoglobin (Hb), glycated hemoglobin (HbA1c), lipid profiles, and hepatorenal functional markers, including serum creatinine, total bilirubin, serum alkaline phosphatase (ALP), and alanine aminotransferase (ALT).
2.2 Diagnostic work-up
Laboratory findings (Table 1) showed that the patient’s FG level was 198 mg/dL, and HbA1c was 9.9 (%). His total cholesterol (TC), low-density cholesterol (LDL-C), high-density cholesterol (HDL-C), and triglyceride (TG) levels were 210, 114, 25, and 220 mg/dL, respectively. His serum creatinine was 1.3, ALP 115, ALT 52, and his serum bilirubin was 1.0. A value of > 200 mg/dL TC is considered borderline high. LDL-C should be < 100 mg/dL, HDL-C should be > 40 mg/dL, and TG should be 120 mg/dL. The physical appearance of a diabetes-prone patient has different characteristics, but risk factors such as obesity, especially in the abdominal area, and a sedentary lifestyle may be obvious and indicate a predisposition to diabetes. There are also skin problems such as dryness or slow wound healing, often accompanied by symptoms such as increased thirst and frequent urination. An individual is categorized as prediabetic if their HbA1c level falls within the range of 5.7% to 6.4%, whereas a diagnosis of diabetes is established when the HbA1c value is 6.5% or above [19, 20]. The patient was, therefore, diagnosed with diabetes and suffered from mild hypercholesterolemia and hypertriglyceridemia. In Bangladesh, recent estimates place the average BMI of the population between 19~20 kg/m2 [21–23], lower than estimates in the USA (27 kg/m2) [24], Japan (23.5 kg/m2) [25], and India (21.7 kg/m2) [26]. The BMI of the person’s family members was between 19 and 20 kg/m2. These BMI values thus indicate that the person in our case was also more overweight. Therefore, his high BMI (26.9 kg/m2) suggested that heavy sitting during sedentary work made him an overweight person. Until then, the patient had neither vision problems nor cardiac complications (Figure 1).
| Parameters | Before Ganotea | After Ganotea |
| Day 0 | Day 45 | Day 90 | DDay45-0 | DDay90-0 |
| Fasting blood glucose (FG) (mg/dL) | 198 | 180 | 177 | -18 | -21 |
| *HbA1c (%) | 9.9 (8.5) | 8.5 (7.9) | 8.4 (7.8) | -1.4 (-0.6) | -1.5 (-0.7) |
| Hemoglobin (Hb) (g/dL) | 14.0 | 14.4 | 15.5 | 0.4 | 1.5 |
| Total cholesterol (TC) (mg/dL) | 210 | 180 | 170 | -30 | -40 |
| Low-density cholesterol (LDL-C) | 114 | 110 | 108 | -4 | -6 |
| High-density cholesterol (HDL-C) | 25 | 27 | 35 | 2 | 10 |
| Triglyceride (TG) | 220 | 215 | 150 | -5 | -60 |
| Serum creatinine | 1.30 | 1.02 | 0.57 | -0.28 | -0.73 |
| Serum alkaline phosphatase (ALP) | 115 | 111 | 107 | -4 | -8 |
| Serum alanine aminotransferase (ALT) | 52 | 58 | 55 | 6 | 3 |
| Serum total bilirubin | 1.0 | ND | 1.04 | – | 0.04 |
Table 1: Effect of Ganoderma lucidum therapy on FG, lipid profile, ALP, ALT, and bilirubin levels of the diabetic patient. Results are the mean of quadruplicate determinations. At each clinic visit, fasting blood was collected twice (at 8 am and 9 am) by venipuncture, centrifuged at 2000 X g and 4°C, and subjected to analysis. Determination was performed in duplicate for each sample. As a result, the above table shows the average of four determinations. Day 0 is defined as the date of the first day the patient donated blood to test the parameters before starting to drink Ganotea. Likewise, day 45 means blood was drawn after drinking Ganotea for 45 days. Day 90 means blood was drawn after drinking Ganotea for 90 days. DDay45-0, the difference in values between day 0 and day 45. DDay90-0, the difference in the value between day 0 and day 90. *HbA1c values given in brackets were determined according to the mathematical equation between HbA1c and FG value [17, 18].
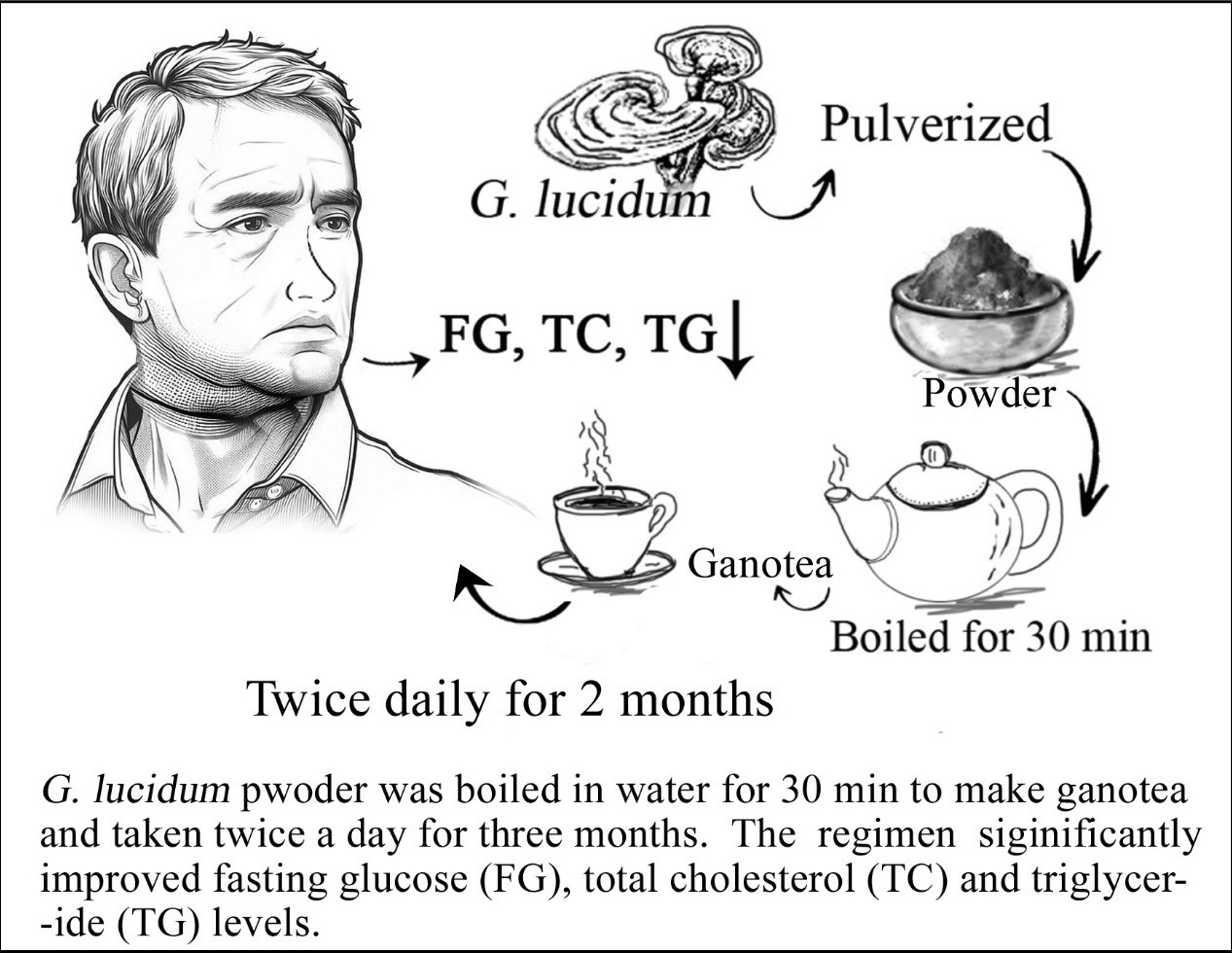 Figure 1: Depicts making Ganotea and its drinking regimen.
Figure 1: Depicts making Ganotea and its drinking regimen.
2.3 Treatment and follow up
However, the patient was very resistant to taking any Western medications for diabetes and dyslipidemia and preferred to ‘wait and see.’ He was also unable to reduce his sedentary time due to his occupational requirements. Regardless of the severity of the disease, the patient initially preferred herbal treatment and was accordingly prescribed G. lucidum powder as a drinking tea. The patient was informed that his FG and other blood parameters would be monitored. A blood test for diabetes was performed 45 days after the patient drank the G. lucidum tea twice a day. Again, the patient returned to the clinic 90 days later to be reassessed for diabetes and dyslipidemia for the third time.
2.4 Outcome
The results of the treatment of G. lucidum hot water extract are also shown in the table (Table 1). 45 days later, the patient’s FG levels decreased from 198 to 180 mg/dL. After 90 days, the FG level dropped to 177 mg/dL. HbA1c levels also decreased, but at the same time, Hb levels increased after both 45 and 90 days of Ganotea consumption (Table 1). After 45 and 90 days of drinking Ganotea, TC levels decreased from an initial value of 210 mg/dL to 180 and 170 mg/dL, respectively. After the prescribed treatment, his TG levels dropped significantly from 220 to 150 mg/dL after drinking Ganotea for 90 days. LDL-C decreased by 6 mg/dL after 3 months, while HDL-C increased from an initial 25 to 35 mg/dL. Hepatorenal functional markers, including creatinine, ALP, ALT, and total bilirubin, were not significantly changed (Table 1). The CBC was also not significantly changed (data not shown).
3. Discussion
The results of the current case report indicate that the patient was type 2 diabetic and suffered from mild hypercholesterolemia and hypertriglyceridemia. Drinking G. lucidum (~4 g/day) hot water extract, Ganotea, for three months, resulted in reductions in FG, TC, and TG levels as well as better HDL-C levels (Table 1). In addition, serum creatinine, ALT, ALP, bilirubin, and CBC were not changed, suggesting that drinking G. lucidum had no adverse effects on liver and kidney function. There were also no discernible corrosive effects on tooth enamel. The patient made no changes to his diet or lifestyle during the three months of taking G. lucidum. After drinking the G. lucidum hot water extract for three consecutive months, our patients’ FG levels decreased from 198 mg/dL to 177 mg/dL, suggesting that the extract had a positive effect on lowering blood sugar levels. Because there is limited human research outside of China, the results of this case show that consumption of G. lucidum hot water extract had beneficial effects in this patient with a sedentary lifestyle, obesity, type 2 diabetes, and dyslipidemia. This provides clinical support for further research into the real-world effects of this supplement. Measurements of hemoglobin A1C (HbA1c) are considered to be far more meaningful data for diabetic prognosis than a single FG. It is the percentage of glycated hemoglobin in total Hb [27]. The population with lower Hb may have higher HbA1c [28] and vice versa, supporting the idea that HbA1c may be associated with Hb levels independently of glycemia. It can also vary by race [28] and gender [29]. Our patients’ Hb levels increased after consuming Ganotea, resulting in a decrease in FG and HbA1c. Therefore, the exact mechanisms still need to be delineated.
In our previous research, G. lucidum has shown tremendous potential benefit in the palliative form of treatment of patients with OSCC. OSCC patients who received G. lucidum had increased appetite, increased tolerance to radiotherapy, better Karnofsky score, improved serum creatinine, ALP, AST, and Hb levels, and decreased serum TNFα and lipid peroxide (LPO) levels, showing that G. lucidum has potential benefits in the palliative form of treatment for patients with OSCC [8]. Similar beneficial effects of G. lucidum were observed in our previous animal and in vitro studies [8–12] with respect to hepatotoxicity, hemolysis, Pb toxicity, and impairments in memory-related brain cognition.
These references emphasize that G. lucidum also has obvious beneficial effects on human diseases and that the effects are not as controversial and/or imaginary as assumed by many modern physicians in various parts of the world but rather beneficial. Here too, the effect is a fact as the results of this case report provide clear evidence that the positive results of numerous previous in vitro and/or animal studies can also be translated into positive effects in human diseases. Although our data provide clear evidence for the antihyperglycemic effects of G. lucidum powder, we are unclear about the mechanisms associated with these beneficial effects.
Several molecular mechanisms by which G. lucidum improves diabetic status have been proposed. For example, polysaccharides from G. lucidum promote the regeneration of partially destroyed beta cells and stimulate insulin secretion [30], improve insulin resistance [31], inhibit postprandial intestinal absorption of glucose [32], increase glycolytic enzymes and/or inhibit gluconeogenesis [33, 34] or glycogenolysis [34] and interfere with carbohydrate digestion by inhibiting glucosidase [35], which overall benefits diabetic. Whatever the mechanism, our case report supports that G. lucidum ameliorates diabetes in humans, and our results were qualitatively consistent with numerous other animal studies [13–15, 30–33] reporting its antidiabetic effects.
Type 2 diabetes is associated with dyslipidemia, suggesting that elevated TC, TG, and LDL-C levels, while low HDL-C levels contribute to secondary complications of diabetes [36]. Our diabetic patient showed a significant decrease in TC and TG after drinking Ganotea, suggesting that our patients’ cardiovascular risk factors improved. The antilipidemic effects were consistent with other studies [30]. It is generally accepted that the reduction in TC is due to decreased activity of the rate-limiting enzyme HMG-CoA reductase (hydroxymethylglutaryl-CoA reductase) or to inhibition of intestinal absorption of dietary cholesterol/or increased cholesterol secretion in the bile, thereby increased excretion of cholesterol through the feces. We do not know which of the above mechanisms contributed more to lowering blood cholesterol levels in our patient or if they all worked together, but these mechanisms may have contributed to the patient’s cholesterol lowering.
In addition, other components found in G. lucidum, including glucans, lectins, triterpenes, ergostane, and lanosterol derivatives, can inhibit HMG-CoA reductase, in particular, β-glucans are the fibrous components of G. lucidum that can interact with lipids and bile salts and thus influence cholesterol absorption and thereby lowering blood cholesterol levels [37, 38]. Currently, there is no clear consensus on how G. lucidum affects TG levels, although G. lucidum has been reported to reduce hypertriglyceridemia [39], probably by increasing beta-oxidation and directing TG into fecal excretion.
4. Conclusion
Finally, taking Ganotea for three months resulted in significant improvement in diabetes and diabetes-related dyslipidemia. Based on this case report and review of current literature, Ganotea may be a useful approach for patients with type 2 diabetes and dyslipidemia who are unwilling to take Western medicines for diabetes. However, further studies on a large population with G. lucidum tea are essential to determine whether it has antihyperglycemic and antidyslipidemic effects. If so, this would be of great benefit to the treatment of diabetics in Bangladesh in the fight against the increasing prevalence and morbidity of diabetes.
Consent
Written informed consent was obtained from the patient for the publication of this case report.
Funding Statement
This research did not receive specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
Acknowledgments
Conceptualization: Hossain S, Alam N; Methodology: Islam KA, Hossain S, Hossain M, Chowdhury BK and Joy SI; Design: Hossain S, Alam N, Hossain M and Chowdhury BK; Collection of data: Islam KA, Hossain S, Hossain M, Chowdhury BK, Joy SI; Analysis: Islam KA, Hossain S, Joy SI; Interpretation of data: Hossain S; Drafting the manuscript: Islam KA, Hossain S, Joy SI; Revising the manuscript: Islam KA and Hossain S. All authors read and approved the revised version of the manuscript.
References
- Afroz A, Alam K, Ali L, et al. Type 2 diabetes mellitus in Bangladesh: a prevalence based cost-of-illness study. BMC Health Serv Res. 2019;19(1):601.
- Cör Andrejč D, Knez Ž, Knez Marevci M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front Pharmacol. 2022;13:934982.
- Oke MA, Afolabi FJ, Oyeleke OO, et al. Ganoderma lucidum: Unutilized natural medicine and promising future solution to emerging diseases in Africa. Front Pharmacol. 2022;13:952027.
- Shin MJ, Chae HJ, Lee JW, et al. Lucidumol A, Purified Directly from Ganoderma lucidum, Exhibits Anticancer Effect and Cellular Inflammatory Response in Colorectal Cancer. Evid Based Complement Alternat Med. 2022;2022:7404493.
- Luangharn T, Karunarathna SC, Dutta AK, et al. Ganoderma (Ganodermataceae, Basidiomycota) Species from the Greater Mekong Subregion. J Fungi (Basel). 2021;7(10):819.
- Benzie IFF, Wachtel-Galor S. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Boca Raton: CRC Press/Taylor & Francis; 2011.
- Wachtel-Galor S, Yuen J, Buswell JA, et al. In: Benzie IFF W-GS, editor. Herbal Medicine: Biomolecular Clinical Aspects. 2nd ed. FL: Boca Raton (FL): CRC Press/Taylor & Francis; 2011.; 2011.
- Hossain M, Bhowmick S, Sarkar M, et al. Effects of Ganoderma Lucidum on palliative care in oral squamous cell carcinoma (OSCC) patients: An evidence of excellent postoperative support for cancer patients. J Dental Med Sci. 2018;17(1):80-91.
- Rahman M, Hossain S. Preventive effect of Ganoderma lucidum on paracetamol-induced acute hepatotoxicity in rats. J Sci Res. 2013;5(3):573-78.
- Hossain S, Bhowmick S, Islam S, et al. Oral Administration of Ganoderma lucidum to Lead-Exposed Rats Protects Erythrocytes against Hemolysis: Implicates to Anti-Anemia. Evid Based Complement Alternat Med. 2015;2015:463703.
- Rahman MA, Hossain S, Abdullah N, et al. Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes) Ameliorates Spatial Learning and Memory Deficits in Rats with Hypercholesterolemia and Alzheimer’s Disease. Int J Med Mushrooms. 2020;22(1):93-103.
- Rahman M, Hossain S, Abdullah N, et al. Ganoderma lucidum Ameliorates Spatial Memory and Memory-Related Protein Markers in Hypercholesterolemic and Alzheimer’s Disease Model Rats. Arch Neurol Neurol Disord. 2020;3(2):117-29.
- Wang F, Zhou Z, Ren X, et al. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis. 2015;14:49.
- Ma HT, Hsieh JF, Chen ST. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry. 2015;114:109-13.
- Zhang Y, Fang X, Wei J, et al. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules. 2022;12(12):1785.
- Allgot B, Gan D, King H. International diabetes federation. Report. Brussels: International Diabetes Federation, ; 2003 2003.
- Sayed A, Alyafei F, De Sanctis V, et al. Translating the HbA1c assay into estimated average glucose values in children and adolescents with type 1 diabetes mellitus. Acta Biomed. 2018;89(S5):22-6.
- HbA1C FS The future of Diabetes Management. In: Calculator HC, editor. 2015.
- Eyth E, Naik R. Hemoglobin A1C. Treasure Island: StatPearls Publishing; 2023.
- d’Emden MC, Shaw JE, Jones GR, et al. Guidance concerning the use of glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus. Med J Aust. 2015;203(2):89-90.
- Shafique S, Akhter N, Stallkamp G, et al. Trends of under-and overweight among rural and urban poor women indicate the double burden of malnutrition in Bangladesh. Int J Epidemiol. 2007;36(2):449-57.
- Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163(12):1138-148.
- Zaman MM, Yoshiike N. Prevalence of overweight defined by body mass index in a rural adult population of Bangladesh. J Health Popul Nutr. 2003;21(2):162-63.
- Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. Int J Obes Relat Metab Disord. 2000;24(7):807-18.
- Tsugane S, Sasaki S, Tsubono Y. Under- and overweight impact on mortality among middle-aged Japanese men and women: a 10-y follow-up of JPHC study cohort I. Int J Obes Relat Metab Disord. 2002;26(4):529-37.
- Sauvaget C, Ramadas K, Thomas G, et al. Body mass index, weight change and mortality risk in a prospective study in India. Int J Epidemiol. 2008;37(5):990-1004.
- Groche D, Hoeno W, Hoss G, et al. Standardization of two immunological HbA1c routine assays according to the new IFCC reference method. Clin Lab. 2003;49(11-12):657-61.
- Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067-072.
- Pan WH, Habicht JP. The non-iron-deficiency-related difference in hemoglobin concentration distribution between blacks and whites and between men and women. Am J Epidemiol. 1991;134(12):1410-416.
- Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 2011;12(9):6135-145.
- Yang Z, Wu F, He Y, et al. A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food & Function. 2018;9(1):397-406.
- Cao H, Guo X. Therapeutic effects of Ganoderma Polysaccharide on type 2 diabetes in rats. 2010;10(12):2256-8. Progress Mod Biomed. 2010;10(12):2256-2258.
- Seto SW, Lam TY, Tam HL, et al. Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine. 2009;16(5):426-36.
- Xiao C, Wu QP, Cai W, et al. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch Pharm Res. 2012;35(10):1793-801.
- Fatmawati S, Shimizu K, Kondo R. Ganoderol B: a potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18(12):1053-055.
- Vijayaraghavan K. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis. 2010;9:144.
- Berger A, Rein D, Kratky E, et al. Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs. Lipids Health Dis. 2004;3:2.
- Sima P, Vannucci L, Vetvicka V. β-glucans and cholesterol. Int J Mol Med. 2018;41(4):1799-1808.
- Viroel FJM, Laurino LF, Caetano ÉLA, et al. Ganoderma lucidum Modulates Glucose, Lipid Peroxidation and Hepatic Metabolism in Streptozotocin-Induced Diabetic Pregnant Rats. Antioxidants (Basel). 2022;11(6):1035.
![]() 2, Joy SI
2, Joy SI![]() 5 and Hossain S
5 and Hossain S![]() *5
*5
