Abstract
People who have celiac disease (CD) are probably more likely to have thyroid disorders. A comprehensive systematic review and meta-analysis were conducted to assess the link between thyroid disorders and CD. Articles were selected from PubMed, Web of Science, Scopus, Ovid, Embase, Cochrane, ProQuest, and Wiley from February 2022 and earlier. A meta-analysis was conducted to evaluate the outcomes, using odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs). The meta-analysis comprised 31 articles with 3310256 participants including 101253 individuals with thyroid disorders. Overall, the frequency of thyroid disease was notably higher in patients with CD compared to the control groups (OR: 3.06, 95% CI: 2.51 – 3.72, P<0.001). The findings of our meta-analysis support the notion that patients with CD are more likely to have autoimmune thyroid disease (ATD) and other thyroid disorders than the control group, thus indicating that regular screening for thyroid disease is necessary for CD patients. Further cohort research is required to investigate the relationship between thyroid disorders and CD.
Keywords
celiac disease, thyroid, autoimmune thyroid disease, thyroid diseases
Abbreviations
CD: celiac disease, OR: odds ratio, CI: confidence interval, ATD: autoimmune thyroid disease, TPO: thyroid peroxidase, TG: thyroglobulin, TSH: thyroid-stimulating hormone, PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR: risk ratio, HR: hazard ratio, NOS: Newcastle-Ottawa Scale, CTLA-4: cytotoxic T lymphocyte antigen-4
1. Introduction
Celiac disease (CD) is an inflammatory autoimmune disorder of the upper small intestine. The disease is triggered by gluten-containing food and environmental factors in genetically predisposed patients with the HLA-DQ2 and HLA-DQ8 [1]. It affects approximately 1% of the global population [2].
CD patients are considered to have more autoimmune diseases than general population [3]. One of the most frequent autoimmune conditions related to CD is autoimmune thyroid disease (ATD). ATD is a group of inflammatory disorders that affect the thyroid gland. The two most common forms of ATD are Graves’ disease and Hashimoto’s thyroiditis [4]. These conditions are characterized by the presence of antibodies such as anti-thyroid peroxidase (TPO), anti-thyroglobulin (TG), and anti-thyroid-stimulating hormone (TSH) receptor antibodies [5]. The risk for the development of these diseases is higher among women compared to men and it also increases with age [6, 7].
Graves’ disease is the most frequent cause of hyperthyroidism. This disorder is characterized by an autoimmune reaction against thyroid antigens, which leads to the secretion of anti-TSH receptor autoantibodies by B cells. These autoantibodies bind to the TSH receptors and chronically stimulate them, leading to chronic secretion of thyroid hormones and thyrotoxicosis [6]. On the other hand, Hashimoto’s thyroiditis can cause hypothyroidism and is clinically characterized by anti-TPO and anti-TG antibodies. In Hashimoto’s thyroiditis, increased infiltration of B and T cells into the thyroid gland causes destruction of follicular cells responsible for the production of thyroid hormones, resulting in hypothyroidism [7].
Although the precise reason for the co-occurrence of CD and thyroid disorders is not yet fully understood, several theories have been suggested. These include genetic predisposition, similar cytokine pathways involved in both disease progression, and impaired absorption of key nutrients due to changes in gut permeability. Such factors may contribute to the higher incidence of these conditions occurring together [8].
Several studies showed this co-occurrence connection. In a cross-sectional study conducted on 288 CD patients, Baharvand et al. [9] reported that thyroid disease was four times more prevalent in CD patients than in controls. Also, Norström et al. [10], in a study of 335 children with CD, claimed that the risk of thyroid dysfunction associated with thyroid autoimmunity is higher for the CD patients than the healthy control. Obaid et al. [4] also investigated 86 CD patients in Iraq from 2018 to 2020. The findings of the study showed a strong association between CD and ATDs. In addition, Bibbò et al. [11] found a significant association between Hashimoto thyroiditis and CD, as the most prevalent autoimmune disease in CD patients than controls. (24.3% vs. 10%).
In 2016, Sun et al. [12] conducted a systematic review and meta-analysis comprising 13 articles with a total of 15,629 CD cases and 79,342 controls. They discovered that the prevalence of thyroid disease was significantly higher in individuals with CD than in the control groups. The odds ratio (OR) was calculated to be 3.08 with a 95% confidence interval (CI) ranging from 2.67-3.56, indicating statistical significance with a P-value of less than 0.001. Interestingly, the analysis revealed that there was no significant difference in the OR between the groups that underwent gluten treatment and those who did not, with an OR of 1.08 and a 95% CI range of 0.61-1.92 (P=0.786). These findings suggest that individuals with CD are more likely to develop thyroid disorders and therefore should undergo regular screening for thyroid disease.
However, there is still a need for a comprehensive and systematic review of the available literature to evaluate this association accurately. Therefore, and to clarify more, we did a new systematic review aimed at evaluating the incidence of thyroid disease in patients with CD after 5 years of the previous meta-analysis study and using 8 different databases for a thorough search. In addition to this primary objective, the study aims to identify any gaps in the existing literature, such as variations in study design and methodological limitations that may affect the validity of the results. By highlighting these gaps, the study can provide insights into future research directions and suggest ways to address these limitations effectively.
2. Materials and Methods
2.1 Eligibility criteria
We designed this study based on the methodology of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [13]. The registration number in the International Prospective Register of Systematic Reviews (PROSPERO) is CRD42021277901.
(Available:https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=277901).
2.2 Inclusion/exclusion criteria
Studies meeting the following criteria were included: 1) human case-control, cohort, and cross-sectional studies reporting on the relationship between thyroid disorders and CD, 2) Reporting of at least one variable showing relative risk effect sizes including risk ratio (RR), OR, and hazard ratio (HR) in both case and control groups. The diagnosis of thyroid disorders in included studies should have been confirmed by serology tests and clinical signs and symptoms. Furthermore, the confirmation of CD should have been based on endoscopy findings, biopsy results, serology tests, and/or HLA DQ2/DQ8 genotyping. Studies that did not include any of this information (including animal studies) were excluded from further analysis.
2.3 Categories of thyroid disorders
Based on a textbook [14] and data extracted from previous original articles, we used four main categories of thyroid disorders for subgroup analysis including hypothyroidism, hyperthyroidism, ATD, and thyroiditis. ATD also had three subcategories including Graves’ disease, Hashimoto’s thyroiditis, and autoimmune hypothyroidism. Some original articles had an emphasis on coexistent hypothyroidism and autoimmunity, but other studies just reported the term ATD; therefore, the association of CD and autoimmune hypothyroidism was also evaluated in the articles that clarified it separately as a subcategory of ATD patients.
Some articles also evaluated and reported the positivity of TPO antibodies and TG antibodies. We extracted and included them in both total and separate analyses.
2.4 Information sources and search strategy
On 28 February 2022, we conducted our systematic search in the following databases to identify relevant articles: PubMed, Web of Science, Scopus, Ovid, Embase, Cochrane, ProQuest, and Wiley. Thorough a three-step process our search keywords and search syntax were designed. First, we extracted the concepts needed for the search following PICO analysis in proportion to the research topic. To accomplish maximum comprehensiveness to recover concepts, we extracted and inserted synonyms, abbreviations, related terms, UK / US spellings singular/plural forms of words, and thesaurus terms in our search syntax. We used the thesaurus MeSH and Emtree for completing keywords. Furthermore, thematic searches on databases were performed. Finally, the preparatory search was conducted and related keywords of main articles on this subject were analyzed. We also enriched and completed the vocabulary. Consequently, the baseline syntax was Thyroid AND (Celiac OR coeliac OR Gluten Enteropath* OR Sprue). Complete search strategies on each database are explained (Supplementary Data 1). Third, two authors hand-scanned the manual reference lists of all studies and relevant systematic reviews to avoid any missing. We also tracked citations for all the included articles.
2.5 Quality and risk of bias assessment
To eliminate duplicates and sift through the studies, all articles gathered from the databases were imported into Endnote software (Version X9; Thompson Reuters Corporation, Toronto, ON, Canada). Initially, screening was carried out by assessing the titles and abstracts to determine the eligible articles. Two reviewers (Y.A. and K.A.) independently reviewed the chosen full-texts to classify them into three groups: relevant, irrelevant, and unsure. A third supervisor reviewer (SA.H.) resolved any discrepancies. Two independent reviewers (N.Z. and H.F.) evaluated the quality and risk of bias assessment of the eligible articles, using an adapted version of the Newcastle-Ottawa Scale (NOS) form specifically designed for case-control studies [15]. The selected studies were then divided into three groups: poor, fair, and good; according to a score obtained from the selection, comparability, and outcome/exposure categories. This score ranged from zero to eight. In case of any disagreements between the two reviewers, a third reviewer (B.S.) was brought in to resolve the issue.
2.6 Data extraction
Four authors (N.Z., H.F., K.A., and Y.A.) extracted the data from each paper based on the list of required variables. We divided the data into three categories: general information, risk of bias assessment, and study setting. The general information category included the first author’s name, year of publication, country, and type of study. The risk of bias assessment category focused on assessing the risk of bias in each study. The study setting category included study duration, sample size, total population recruited in each study, age group and age range, sex, the definition of CD, source of the data, diagnostic methods, each thyroid disorder, P-value, and ethical approval. To fix missing items or provide additional necessary data, we sent an e-mail to the corresponding authors of those articles, if possible.
2.7 Data synthesis and analysis
We used STATA software, version 12, to calculate pooled OR and its corresponding 95% CIs for each study related to the chance of developing thyroid disease in patients with CD. The combined OR was presented in a forest plot. Heterogeneity among studies was measured using the Q Cochrane test and I2 index. A value less than 50% was considered moderate heterogeneity, while a value above 50% was considered severe heterogeneity. According to the existence of heterogeneity in the methodology of the studies, at moderate levels of heterogeneity, a fixed-effect model was used and at severe levels of heterogeneity, a random-effect model was used to combine data in meta-analysis. For finding the cause(s) of heterogeneity, and to investigate the effect of the type of studies and their quality on the research results subgroup and sensitivity analysis was used. Subgroup analysis evaluated factors including the thyroid outcome, type of study, and the risk of bias assessment. In order to evaluate for the presence of any potential publication bias, Begg’s and Egger’s tests were performed to assess asymmetry in the funnel plot. A P-value of less than 0.05 was deemed to be significant. To further investigate the impact of individual studies on the overall pooled OR, a sensitivity analysis known as the one-out remove method was utilized. Because two different populations in two different time periods were reported in the Grode et al. [3] 2018 study, the data of this study was included separately in the analysis, as data I and II.
3. Results
3.1 Search results
In our primary search, 6304 papers were found in the mentioned databases. After removing duplicates, 4186 citations remained for the title and abstract screening. Based on the inclusion and exclusion criteria, 4114 papers were excluded and 72 full-text articles were used for screening. Out of the available full-text studies, 31 were deemed eligible and included in both the systematic review and meta-analysis. The reasons for excluding other full-text studies are listed in the figure (Figure 1).
3.2 Characteristics of the included articles
The table (Table 1) shows the detailed description of key characteristics for the 31 included studies. There were 3310256 participants in total, of these, 101253 were in the thyroid group and 3209003 were in the non-thyroid group. Among the studies, Dhalwani et al. [16] had the highest study population with 2426224 participants, including 6506 cases and 2419718 controls. All studies were published between 1998 and 2022; furthermore, the data of studies were from the Europe and Asia continents, as well as the USA. In 47.06% of the studies, the thyroid was defined as autoimmune. As a result, the NOS assessment showed that only 67.74% of the studies had good quality.
| First author | Year | Country | Type of study | Definition of CD | Thyroid outcome | Risk of bias assessment | Case-number | Control-number |
| Snook | 1989 | England | Case-control | Medical record | Hypothyroidism & Hyperthyrodism | P a | 148 | 300 |
| Sategna-Guidetti | 1998 | Italy | Case-control | Serology & endoscopy | ATD b | G c | 185 | 170 |
| Velluzzi | 1998 | Italy | Case-control | Serology & endoscopy | TPO d-Ab e | G | 47 | 91 |
| Kowalska | 2000 | Poland | Case-control | IgA-EmA test | Antithyroid antibodies (TMA g and/or ATG h and/or anti-TPO) | G | 34 | 28 |
| Toscano | 2000 | Italy | Case-control | Endoscopy | TPOAbs | F i | 44 | 40 |
| Ventura | 2000 | Italy | Case-control | Serology & endoscopy | TPOAbs | G | 90 | 90 |
| Hakanen | 2001 | Finland | Case-control | Unknown | ATD & Autoimmune hypothyroidism & Subclinical autoimmune thyroiditis & Graves’ disease | G | 79 | 184 |
| Sategna-Guidetti | 2001 | Italy | Case-control and prospective cohort | Serology & endoscopy | ATD | G | 422 | 605 |
| Ansaldi | 2003 | Italy | Case-control and prospective cohort | Endoscopy | ATD | G | 343 | 199 |
| da Rosa Utiyama | 2005 | Brazil | Case-control | Clinical criteria & endoscopy & serology | thyroid microsome antibody positivity | F | 76 | 97 |
| Selimoglu | 2005 | Turkey | Case-control | Serology & endoscopy | Hypothyroidism & Hyperthyroidism & positive for thyroid microsome antibodies & positive for antithyroglobulin antibodies | G | 77 | 40 |
| Guariso | 2007 | Italy | Cohort | | Hashimoto’s thyroiditis & ATD | G | 267 | 220 |
| Elfstrom | 2008 | Sweden | Case-control and prospective cohort | Medical record | Hypothyroidism & Thyroiditis & Hyperthyrodism | P | 14021 | 68068 |
| Naiyer | 2008 | US | Case-control | Serology & endoscopy | TPO-Ab, TG-Ab | G | 40 | 40 |
| Neuhausen | 2008 | US | Case-control and prospective cohort | Serology & endoscopy | Hypothyroidism | F | 408 | 1272 |
| Toumi | 2008 | Tunisia | Case-control and prospective cohort | Serology & endoscopy | TG-Ab, TPO-Ab, TRAb | G | 104 | 189 |
| Caglar | 2009 | Turkey | Cross-sectional | Endoscopy &/or serology | anti-TPO positivity & anti-TG positivity | G | 31 | 29 |
| Carlsson | 2009 | Sweden | Prospective cohort | Serology | TPO Ab positivity | G | 207 | 1151 |
| Garud | 2009 | USA | Case-control | Medical record | ATD | P | 600 | 200 |
| Meloni | 2009 | Italy | Case-control and prospective cohort | Serology & endoscopy | ATD | G | 324 | 8040 |
| Diamanti | 2011 | Italy | Case-control and prospective cohort | Serology & endoscopy | ATD | G | 545 | 622 |
| Ludvigsson | 2011 | Sweden | Case-control and prospective cohort | Endoscopy | ATD | F | 7843 | 38966 |
| Elli | 2012 | Italy | Case-control | Anti-tTG j Ab test & Positive response to GFD k | Hashimoto thyroiditis and Grave’s disease | G | 1015 | 848606 |
| Ban | 2014 | UK | Population-based cohort | Medical record | Thyroid disorders | P | 1880 | 560452 |
| Dhalwani | 2014 | UK | Cohort | Medical record | Thyroid disorders | P | 6506 | 2419718 |
| Schuppan | 2014 | Germany | Case-control | Serological and genetic investigations | ATD | P | 101 | 132 |
| van der Pals | 2014 | Sweden | Case-control and prospective cohort | Serology & endoscopy | TPO Abs | G | 335 | 1695 |
| Canova | 2016 | Italy | Prospective matched cohort | Endoscopy & hospital admission & copayment exemption | Autoimmune hypothyroidism & hyperthyroidism | G | 1215 | 6075 |
| Bibbò | 2017 | Italy | Case-control | Serology & endoscopy | Hashimoto thyroiditis | G | 255 | 250 |
| Grode I | 2018 | Denmark | Nation-wide population-based study | Medical record | Graves’ disease | G | 4171 | 43774 |
| Grode II | 2018 | Denmark | Nation-wide population-based study | Medical record | Graves’ disease | G | 10285 | 104928 |
| Norström | 2018 | Sweden | Case-control | Serology | TPO Abs | G | 309 | 1665 |
| Khan | 2019 | USA | Population-based studie | Medical record | Hashimoto thyroiditis & Grave disease | G | 249 | 498 |
| Baharvand | 2020 | Iran | Comparative cross-sectional | Serology & endoscopy | Hypothyroidism & Hyperthyroidism & TPO Abs | P | 288 | 250 |
| Tiberti | 2020 | Italy | Case-control | Serology & endoscopy | TPO Abs | P | 92 | 237 |
Table 1: Main characteristics of the included studies in the meta-analysis on the thyroid disorders infection and celiac disease (CD). A: poor; B: autoimmune thyroid disease; C: good; D: thyroid peroxidase antibody; E: antibody; F: IgA-endomysial antibodies; G: thyroid microsomal autoantibodies; H: anti-thyroglobulin antibodies; I: fair; J: tissue transglutaminase; K: gluten-free diet.
The meta-analysis demonstrated that there is a significant relationship between CD and thyroid disorders (pooled OR: 3.06; 95% CI: 2.51 – 3.72; P ≤ 0.001). Forest plot of the frequency of thyroid disorders in CD patients compared with the control group is shown in the figure (Figure 2).
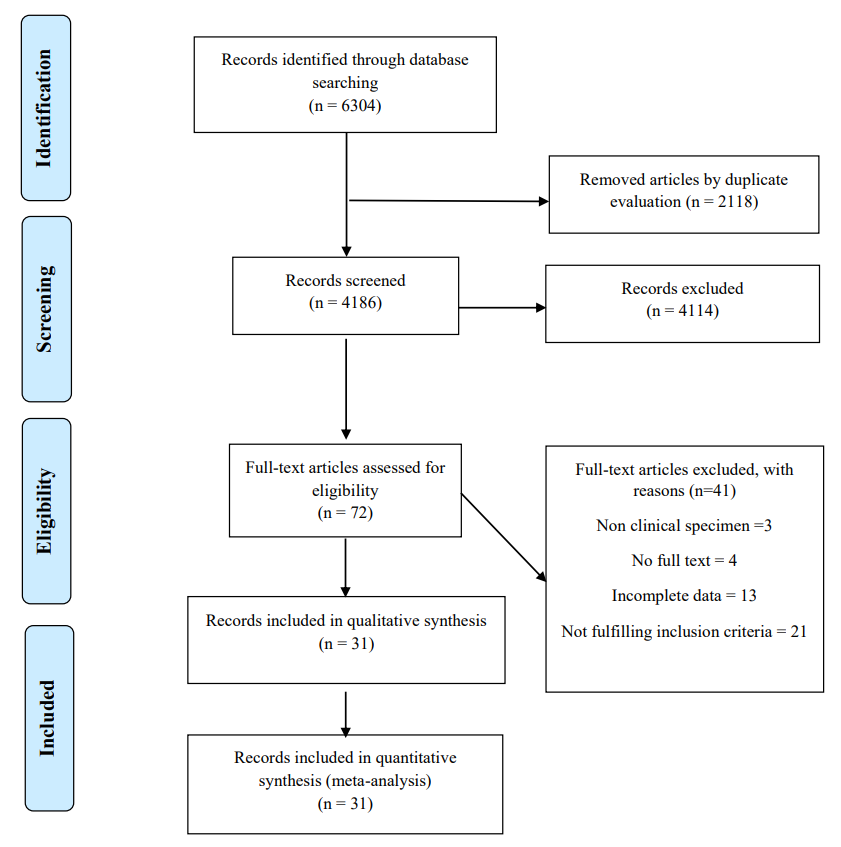 Figure 1: The process of study selection.
Figure 1: The process of study selection.
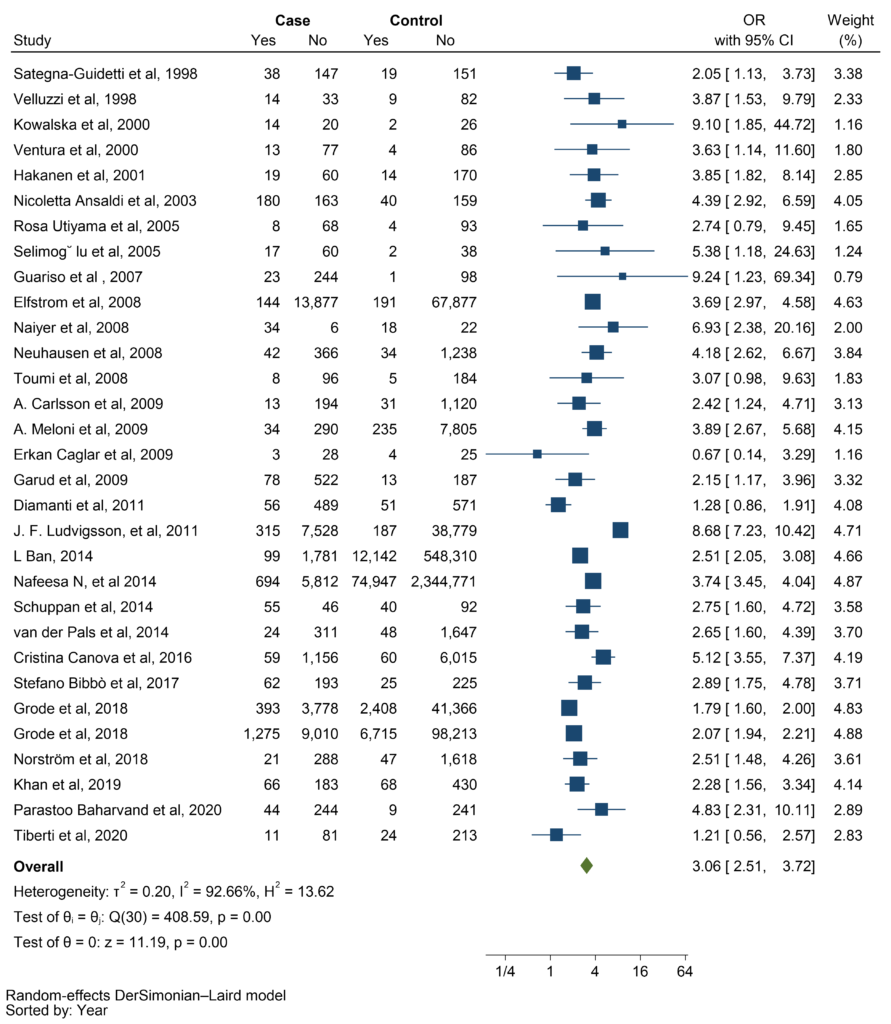 Figure 2: Forest plot illustrating the strong association between celiac disease (CD) and thyroid disorders (p < 0.001, pooled OR: 3.06; 95% CI: 2.51-3.72).
Figure 2: Forest plot illustrating the strong association between celiac disease (CD) and thyroid disorders (p < 0.001, pooled OR: 3.06; 95% CI: 2.51-3.72).
3.3 Subgroup analysis
To assess the heterogeneity of the studies, we conducted multiple subgroup analyses based on three factors: types of thyroid disorders, types of studies, and quality of studies. The amount of heterogeneity did not change significantly within the subgroup analyses; therefore, they cannot be a source of the heterogeneity. As shown in the figure (Figure 3), In the subgroup analysis by study type, cross-sectional studies had a lower pooled OR than case-control and cohort studies (OR: 2.04 vs OR: 3.09 and OR: 3.48, respectively).
Based on the NOS assessment, most studies had good scores. The pooled OR for fair studies was higher than for poor and good studies (OR: 5.36 vs OR 2.98 and OR: 2.76 respectively) (Figure 4).
Furthermore, the analysis showed a significant relationship between CD and ATD (OR: 2.96, CI: 2.32 – 3.78, P ≤ 0.001) (Figure 5).
Random effects meta-analysis was conducted for interaction in planned subgroups and adjusted common effects (Figure 6).
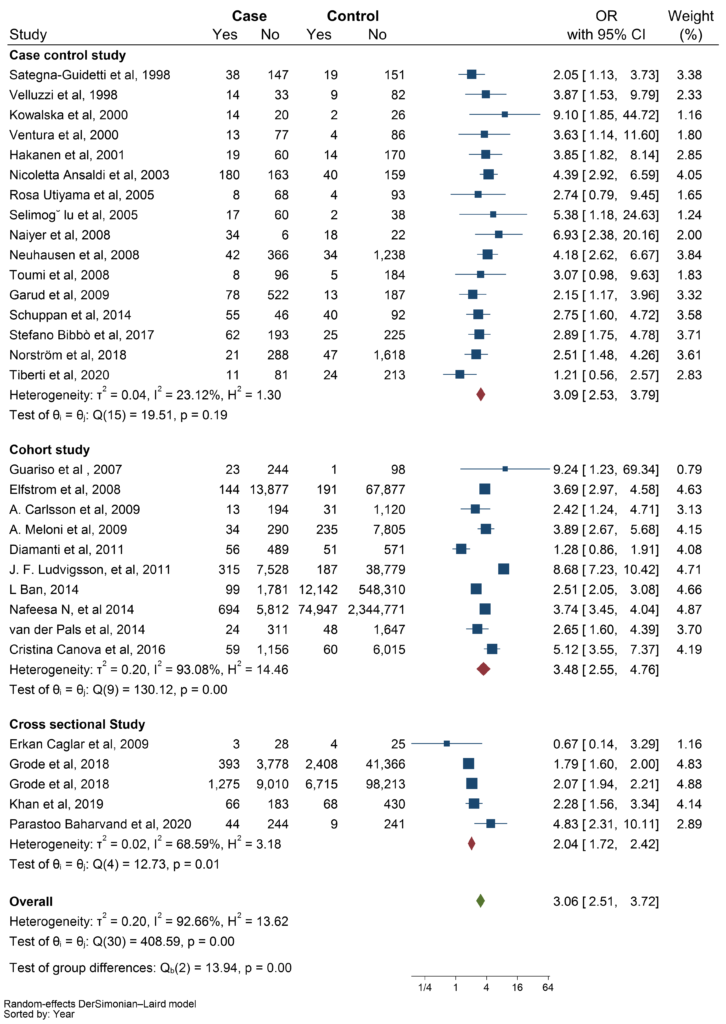 Figure 3: Demonstration of subgroup analyses based on type of study.
Figure 3: Demonstration of subgroup analyses based on type of study.
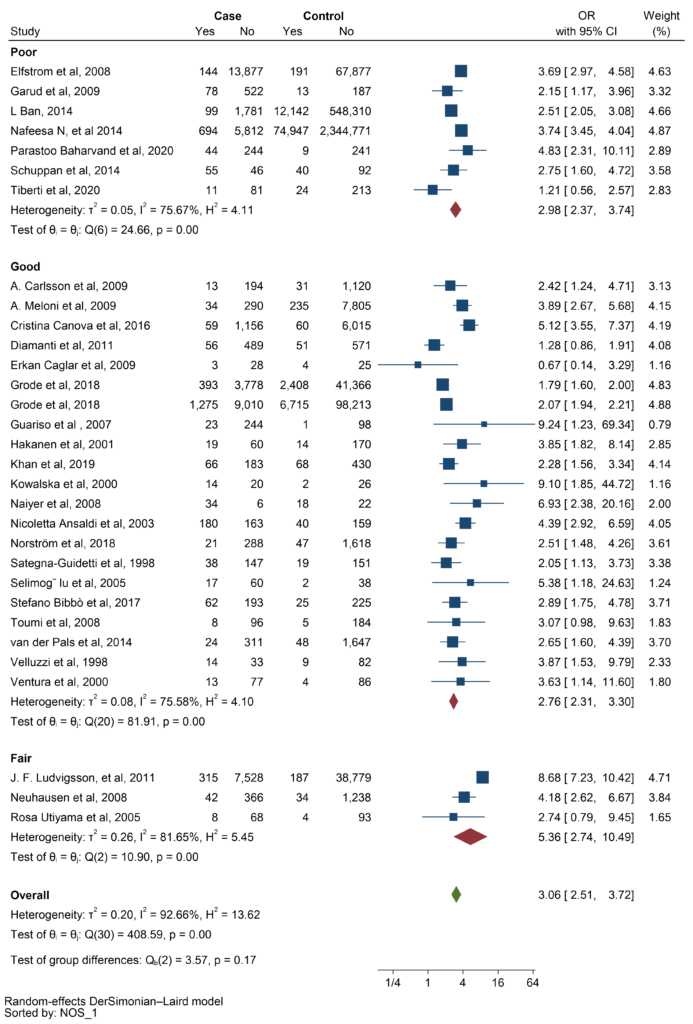 Figure 4: Subgroup analysis based on Newcastle-Ottawa Scale (NOS) assessment.
Figure 4: Subgroup analysis based on Newcastle-Ottawa Scale (NOS) assessment.
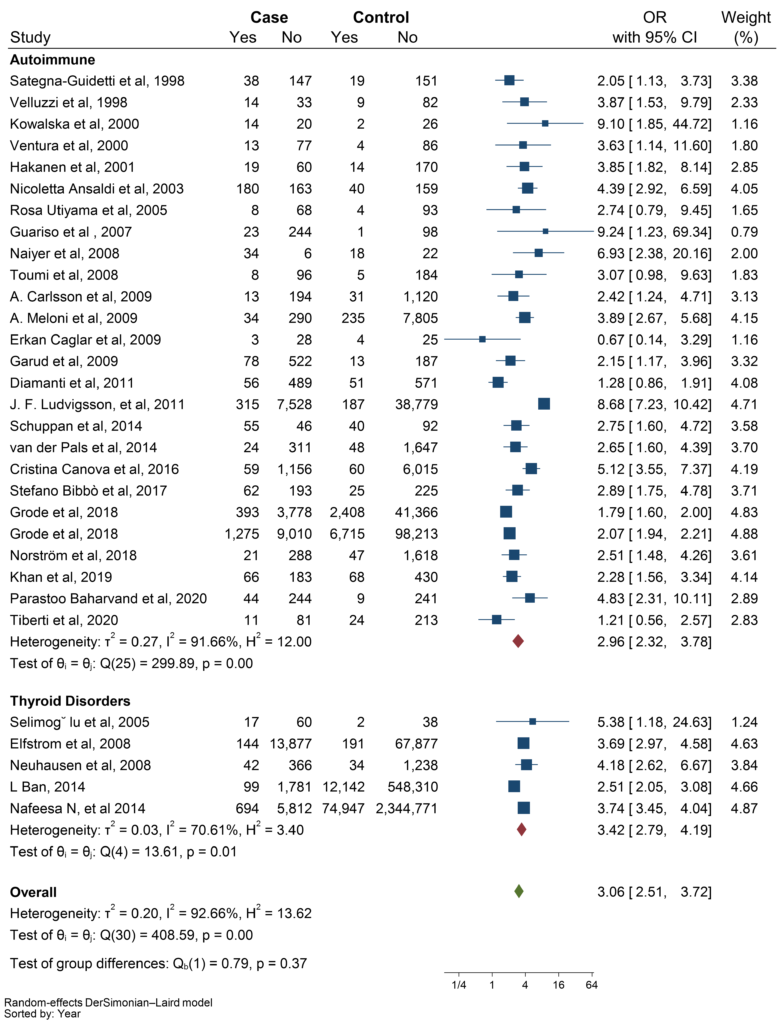 Figure 5: Subgroup analysis based on autoimmune thyroid disease (ATD) and other thyroid disorders.
Figure 5: Subgroup analysis based on autoimmune thyroid disease (ATD) and other thyroid disorders.
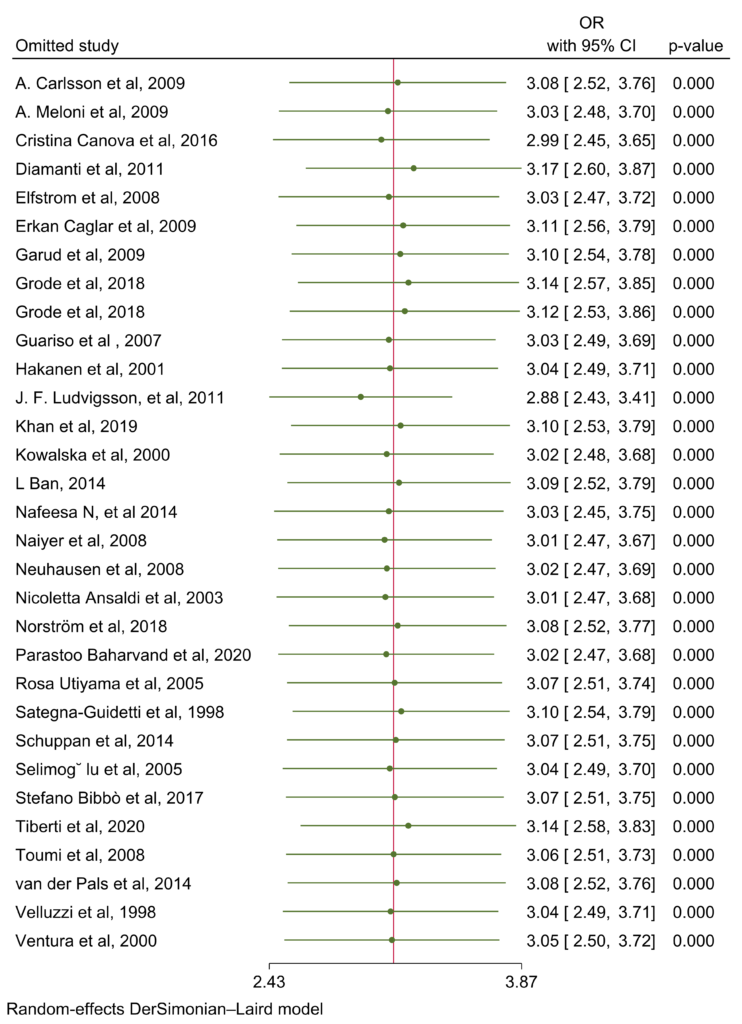 Figure 6: Random effects of all 31 articles.
Figure 6: Random effects of all 31 articles.
3.4 Publication bias
The results of Begg’s and Egger’s tests indicate that the funnel plot does not exhibit significant asymmetry, suggesting the absence of publication bias in the data. This inference is supported by the p-values obtained from Begg’s test (0.75) and Egger’s test (0.61) (Figure 7).
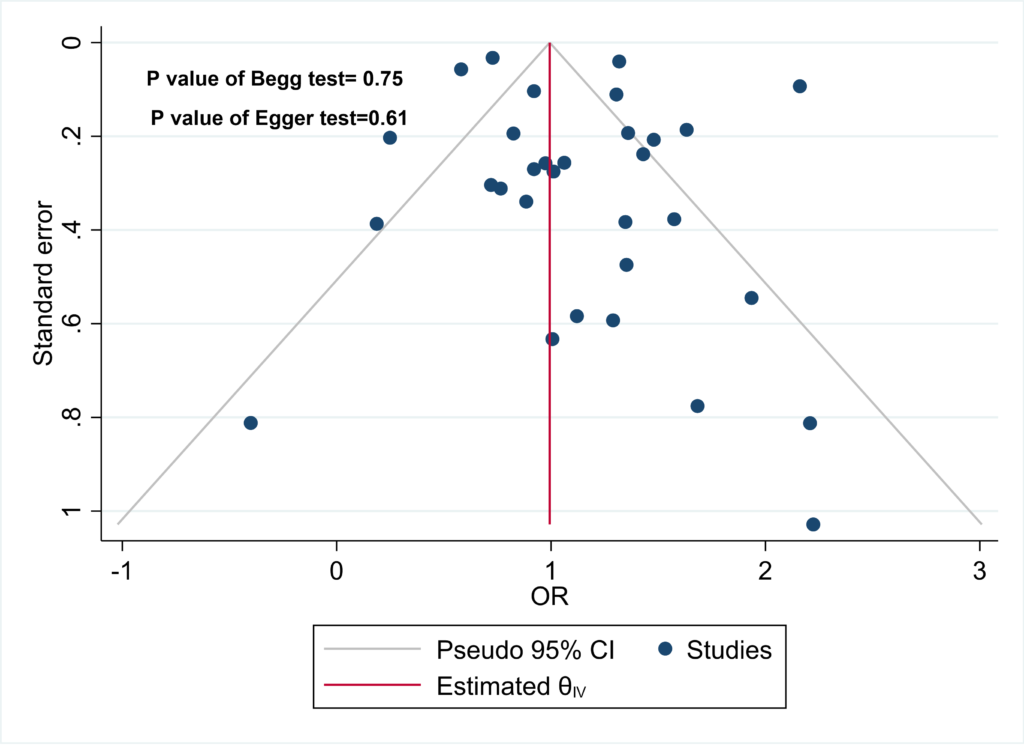 Figure 7: Begg’s test (p = 0.75) and Egger’s test (p = 0.61) results confirm the absence of significant publication bias.
Figure 7: Begg’s test (p = 0.75) and Egger’s test (p = 0.61) results confirm the absence of significant publication bias.
4. Discussion
To evaluate the risk of thyroid disorders in patients with CD, several case-control and cohort studies have been conducted or are currently underway. However, these studies have varying methodologies and sample sizes, and their results are inconclusive. To address this issue, a systematic review and meta-analysis were conducted to combine all relevant studies and establish strong evidence of any association between CD and thyroid disorders. After analyzing the findings of 31 studies, we have demonstrated that individuals with CD are at a significantly higher risk of developing thyroid disorders compared to those in the control population. In fact, the risk is found to be 3.06 times greater for CD patients.
The pathophysiologic reasons why thyroid disorder is more common with CD are still under discussion and are not well known. Regarding that, there are several suggested mechanisms. One of them is related to genetic factors. Studies have shown that human leukocyte antigen (HLA) is overexpressed in CD. Specifically, HLA-DQ2 and DQ8 have been found to be associated with ATDs, particularly Hashimoto’s thyroiditis. A meta-analysis conducted on this topic revealed that Hashimoto’s thyroiditis was more than three times as prevalent in CD patients compared to those without CD. The gene that encodes cytotoxic T lymphocyte antigen-4 (CTLA-4) and ATD. CTLA-4 is located in the HLA region and is also associated with CD, therefore can have a strong connection with thyroid autoimmune diseases [17–19].
In CD, patients suffer from malabsorption of nutrients such as iron, selenium, and vitamin D, due to chronic inflammation and villous atrophy. Iron deficiency worsens thyroid dysfunction due to the decreased activity of the heme-dependent TPO, which relates to ATD (e.g., Hashimoto’s thyroiditis and Graves’ disease) [20]. Another involved mechanism is the deficiency of selenium. This deficiency can modulate the expression of selenium proteins, resulting in inflammation and mucosal damage. Selenium deficiency can negatively impact thyroid function, since selenium proteins act as antioxidants and regulate redox status and thyroid hormone metabolism in thyrocytes. The selenium proteins are also involved in cell growth and apoptosis [21]. The mechanism related to vitamin D deficiency and thyroid disorder can be explained as follows: due to vitamin D deficiency, the suppression of T cells is disrupted, resulting in an increase in the release of inflammatory cytokines. This leads to the destruction of thyroid tissue and ATD [22].
Additionally, tTG-2 IgA antibodies have also been proven to react with thyroid tissue, which may contribute to thyroid disease in CD. Naiyer et al. [23] observed a positive relation between tTG-2 IgA and anti-TPO antibody titers, which indicated the relationship between CD and the presence of organ-specific antibodies against thyroid tissue.
A meta-analysis study conducted in 2016 found that thyroid disorders are three times more prevalent among CD patients compared to those in the control group (OR 3.08, 95% CI 2.67-3.56; P<0.001). In their subgroup analysis for hyperthyroidism by three original articles, they demonstrated that hyperthyroidism has no significant association with CD. However, when a subgroup analysis was conducted using five articles in the present study, it revealed that CD patients have a two-fold risk of developing hyperthyroidism, similar to the impact of the other types of thyroid disorders. On the other hand, our subgroup analysis for hypothyroidism was consistent with the last meta-analysis. The evaluation revealed that the occurrence of hypothyroidism in CD patients was significantly higher when compared to individuals in the control groups (OR 3.38, 95% CI 2.73-4.20, P < 0.001) [12].
After the final search, we reached 18 articles more than the last systematic article. According to new articles and additional data, the categories of thyroid disorders were increased in our study and we analyzed them in separate subgroups to reach their specific OR. We also examined the studies according to groups of the quality of the studies based on NOS assessment and types of studies; to determine the possible discrepancies. Fortunately, no discrepancies were detected. Finally, all subgroup analyses demonstrated a strong effect of CD on the occurrence of different thyroid disorders.
However, there are some limitations in this study. Some original articles specified that the patients had euthyroid autoimmune disease or autoimmune hypothyroidism, but other articles did not mention that. We suggest for future original articles to distinguish the type of autoimmunity. The other limitation is the low number of studies from Asia, and the lack of research from Africa, South America, and Oceania. Thus, the results emphasize the population in America and Europe. Evaluating other ethnicities is helpful for further studies.
5. Conclusion
Our systematic review and meta-analysis of 34 original articles including 102545 CD patients show that they are at three to four-fold risk of thyroid disorders, especially autoimmune hypothyroidism. We suggest the design of a cohort study with a large sample size of CD patients for a long-time follow-up to evaluate any possible thyroid disorders. Furthermore, based on the findings, it is essential to conduct routine screening for thyroid disorders in individuals with CD.
Authors’ Contributions
N.Z.: supervise the project, data extraction, and drafting of the article. H.F.: helped supervise the project, data extraction, and drafting of the article. B.S.: conception and design, revision of the article. SA.H.: supervise the project, and review the screening of the articles. G.S.: data analysis, revision of the article. Y.A.: screening the articles, data extraction. M.M.: search strategy and writing syntax, revision of the article. K.A.: screening the articles, data extraction. All authors discussed the results and contributed to the final manuscript.
Availability of Data and Materials
All data generated or analyzed during this study are available.
Competing Interests
The authors declare no competing interests.
Supplementary Material
References
- Barker JM, Liu E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr. 2008;55:349-65.
- Mearns ES, Taylor A, Boulanger T, et al. Systematic Literature Review of the Economic Burden of Celiac Disease. Pharmacoeconomics. 2019;37(1):45-61.
- Grode L, Bech BH, Jensen TM, et al. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur J Gastroenterol Hepatol. 2018;30(1):83-91.
- Obaid KH, Murshd MM, Ali NH. Cd Coexist With Autoimmune Thyroid Diseases. EJMCM. 2020;7(9):682-86.
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252-65.
- Antonelli A, Ferrari SM, Ragusa F, et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101387.
- Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19(10):102649.
- Starchl C, Scherkl M, Amrein K. Celiac Disease and the Thyroid: Highlighting the Roles of Vitamin D and Iron. Nutrients. 2021;13(6):1755.
- Baharvand P, Hormozi M, Aaliehpour A. Comparison of thyroid disease prevalence in patients with celiac disease and controls. Gastroenterol Hepatol Bed Bench. 2020 Winter;13(1):44-49.
- Norström F, van der Pals M, Myléus A, et al. Impact of Thyroid Autoimmunity on Thyroid Function in 12-year-old Children With Celiac Disease. J Pediatr Gastroenterol Nutr. 2018;67(1):64-8.
- Bibbò S, Pes GM, Usai-Satta P, et al. Chronic autoimmune disorders are increased in coeliac disease: A case-control study. Medicine (Baltimore). 2017;96(47):e8562.
- Sun X, Lu L, Yang R, et al. Increased Incidence of Thyroid Disease in Patients with Celiac Disease: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(12):e0168708.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Nyström E, Berg GEB, Jansson SKG, Torring O, Valdemarsson SV. Springer Berlin Heidelberg; 2011.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
- Dhalwani NN, West J, Sultan AA, et al. Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology. 2014;147(6):1267-274.e1.
- Hunt KA, McGovern DP, Kumar PJ, et al. A common CTLA4 haplotype associated with coeliac disease. Eur J Hum Genet. 2005;13(4):440-44.
- Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003;31(1):21-36.
- King AL, Moodie SJ, Fraser JS, et al. Coeliac disease: investigation of proposed causal variants in the CTLA4 gene region. Eur J Immunogenet. 2003;30(6):427-32.
- O’Kane SM, Mulhern MS, Pourshahidi LK, et al. Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutr Rev. 2018;76(6):418-31.
- Sanmartín C, Plano D, Sharma AK, et al. Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci. 2012;13(8):9649-672.
- Mackawy AM, Al-Ayed BM, Al-Rashidi BM. Vitamin d deficiency and its association with thyroid disease. Int J Health Sci (Qassim). 2013;7(3):267-75.
- Naiyer AJ, Shah J, Hernandez L, et al. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid. 2008;18(11):1171-178.
![]() 1, Norouzi Z1, Besharat S
1, Norouzi Z1, Besharat S![]() *1, Shirzad-Aski H2, Ghorbani S3, Mohammadi M
*1, Shirzad-Aski H2, Ghorbani S3, Mohammadi M![]() 1, Yadegari A4 and Kalhori A
1, Yadegari A4 and Kalhori A![]() 5
5






