Abstract
Background: Clinical and diagnostic challenges are due to the result of a variable presentation of neonatal sepsis and bloodstream infections (BSIs) and uncertain disease epidemiology in children below five years of age. Although the criteria for achieving an adequate blood culture specimen in adults have been well described, there is much more equivocation in the pediatric population, especially for the under-five-year age group. Therefore, the present study is designed to evaluate the etiological profile of BSI among under-five children by the automated BACTEC systems.
Materials and Methods: All blood culture samples received in the Department of Microbiology for culture falling in the age group were included in the study for a period of one year from 01 July 2015 to 30 June 2016 using the BD BACTEC FX except in the exclusion criteria. The blood culture was observed in the BD BACTEC FX system for at least five days before being reported as sterile.
Results: A total of 1533 samples in the age group of < 5 years and suspected of BSIs were received in the Department of Microbiology, Indira Gandhi Medical College (IGMC), Shimla. Among them, 963 (62.8%) were males, while 570 (37.2%) were females. Among the total of 1533 samples, 604 (39.40%) were found positive in culture, 898 (58.57%) were negative, and 31 (2.02%) were contaminants. Among the 604 positive cases, 390 (64.6%) were men, while 214 (35.4%) were women. S. aureus was the highest among the gram-positive isolates 98 (16.22%), followed by coagulase-negative Staphylococcus, group B Streptococcus, and S. pneumoniae. Among the gram-negative organisms, E. coli 80 (13.24%) was isolated mostly followed by K. pneumoniae 70 (11.58%), P. aeruginosa 68 (11.25%), Salmonella typhi 50 (8.27%), Citrobacter koseri 18 (2.98%), Acinetobacter baumannii 12 (1.98%) and a group of organisms without fermentation 86 (14.23%).
Conclusion: There was quite high positivity in culture in the preschool group. Positivity was significantly high in males as compared to females. It is essential to administer appropriate and synergistic antimicrobial agents empirically early and appropriately for treating children under five-year age with BSI.
Keywords
bloodstream infections, culture, BACTEC, preschool children
Abbreviations
BSIs: bloodstream infections, IGMC: Indira Gandhi Medical College, CoNS: coagulase-negative staphylococci, HIV: human immunodeficiency virus, ESBLs: extended-spectrum beta-lactamases
1. Introduction
Neonatal sepsis is defined variably globally based on clinical signs and symptoms and laboratory diagnosis, which makes the study of this common but devastating condition very difficult. Developing countries or countries, especially in Asia, Africa, and America, have an inordinate burden of bacterial bloodstream infections (BSIs) as compared to developed countries. Neonatal sepsis is a huge contributor to morbidity and mortality of infants and mostly among under-five age group children world over, and increasing health care costs for the patient’s families [1–3]. The significant results of BSIs are such that the treatment initiated is generally empirical due to deferments in the culture. In the developing world, this is further made worse by the reduced availability of well-resourced microbiology facilities [4]. A study on the cost-effectiveness of BSI surveillance for the management of sepsis in low-resource-based settings conducted recently by Penno et al. [5], opined that antibiotic prescription if based on antibiogram, can save more than 550 lives per 100,000 patients. The finding suggests that it is very crucial to monitor emerging resistance trends at the local level to help and support clinical management in taking decisions for a better therapeutic outcome. Empirical antimicrobial therapy used on such occasions is usually based on international recommendations and is not guided by local susceptibility patterns. Knowledge of the local antibiotic resistance profile of bacteria increases the chances of selecting effective and appropriate empirical treatment initiation [2]. The increase in antibiotic resistance globally means that the chances of inadequate therapy in BSI are increased substantially, with greater chances of poor therapeutic outcomes [7, 8]. Data mainly from high-income countries suggests that 50.9 million cases of sepsis occur worldwide each year, with a capacity to cause 5.3 million deaths annually [6]. The identification of BSIs and further reporting their antibiotic sensitivity is the most crucial and critical task performed by the clinical microbiology laboratory. However, the criteria and guidelines for getting an adequate blood culture specimen in adults have been well documented and defined, but that is not the case in pediatric populations [1]. BSIs and sepsis are major challenges in medicine. The problem is more severe in neonates and children under the age of five years with high-grade fever and no apparent or localized focus of infection. They cause life-threatening emergencies that lead to substantial morbidity and mortality. Changing patterns of isolates, increasing rates of resistance, and wide application of new medical technologies, such as rampant usage of indwelling devices, are significant contributors to changing the epidemiology and outcome of BSI [2]. Sepsis is a well-established cause of morbidity and mortality even among adults, and more especially among neonates, infants, and under-five age group children. However, the incidence of sepsis in term and late preterm infants is low, and the potential for serious adverse outcomes is of such grave consequence that parents of the children and attending doctors and caregivers should not take lightly any signs and symptoms for evaluation, diagnosis, and treatment of possible sepsis in neonates, infants, and the under-five age group.
Therefore, it is important to continually review and update the epidemiology of BSIs, concerning antibiotic susceptibility patterns of common pathogens, so that prompt and appropriate empirical treatment of patients may be instituted at the earliest. BSIs are a major cause of morbidity and mortality worldwide and are poorly documented [9]. Illness associated with bacteremia ranges from self-limiting infection to life-threatening sepsis, i.e., mortality ranging from 20–50 % [10].
BSIs are associated with an increased length of hospitalization and are a financial burden on the patients. Improved clinical microbiology services and the evaluation of empirical treatment guidelines are very important in the management of patients with BSIs and could contribute to a better outcome [11]. Blood cultures remain the mainstay of laboratory diagnosis of BSIs in infants and children. Recovery of an infectious pathogen is confirmed by the diagnosis of bacteremia and allows antimicrobial susceptibility testing specifically for treating the organism to reduce hospital stay and duration. A negative blood culture is just as important, as it rules out cases of bacteremia and prompts the continued investigation of other infectious or noninfectious etiologies or the discontinuation of unnecessary empirical antimicrobial therapy [12]. Staphylococcus aureus is one of the leading causes of both community-associated and healthcare-associated invasive infections in children. S. maltophilia is a multidrug-resistant gram-negative bacillus, an opportunistic pathogen, particularly among hospitalized patients. S. maltophilia infections have been associated with increased morbidity and mortality in immunosuppressed and debilitated individuals.
Indira Gandhi Medical College (IGMC), Shimla, is a tertiary care hospital, and patients are treated with above mentioned clinical conditions both in the outpatient department (OPD) and inpatient department (IPD) and generally require blood culture to establish the etiological diagnosis, especially in children under five years of age. Most studies related to the laboratory diagnosis of BSI focus on the adult population. Therefore, the present study is designed to evaluate the etiological profile of BSI among children under five years of age using the automated BACTEC systems.
2. Aim and Objective
To determine the etiological profile of BSI among children under five by the automated BACTEC systems.
3. Materials and Methods
3.1 Study design
Prospective observational study.
3.2 Study setting
Department of Microbiology, Indira Gandhi Medical College and Hospital, Shimla, India.
3.3 Study period
One year from July 2015 to June 2016.
3.4 Inclusion criteria
- All the blood culture samples received in the Department of Microbiology for blood culture by the BD BACTEC FX system from the under-five year age group.
- Parents of the patient or attendants of the patients willing to study with informed or written consent.
3.5 Exclusion criteria
- Parent or attendant of the patient is not willing to study.
- Blood cultures show contamination, which is the growth of more than three different types of colonies on a medium.
3.6 Methodology
All blood culture samples received in the Department of Microbiology for culture in the age group were included in the study except those in the exclusion criteria. The blood culture vials were observed in the BD BACTEC system for a minimum of 5 days before reporting as sterile. Each bottle has a sensor that can detect the increase in Co2 produced by the growth of microorganisms. The sensor monitors every 10 minutes for an increase in fluorescence, which is proportional to the amount of Co2 present. A positive bottle will be sub-cultured on blood agar and MacConkey agar plates. Following the subculture on solid media from each positive bottle, a smear will be prepared for gram staining from that blood culture bottle. The gram-stained smear will be examined for the presence of microorganisms, and the presumptive report will be transmitted to respective departments. All bacterial isolates will be identified using standard biochemical identification methods like indole, methyl red, urease, citrate, triple sugar iron, and mannitol fermentation. Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Streptococcus pneumoniae ATCC 49619, and Pseudomonas aeruginosa ATCC 27853 were inoculated along with the test organisms. All positive isolates were stocked.
3.7 Antibiotic susceptibility test
The antibiotic sensitivity test was performed by diffusion of discs as per CLSI guidelines. The antimicrobial susceptibility pattern was determined by using the standard Kirby-Bauer disk diffusion method. The susceptibility of isolated organisms was tested to antimicrobial agents using Mueller Hinton agar. Drug-resistant strains in screening were processed for the detection of methicillin-resistant Staphylococcus aureus (MRSA) strains and extended-spectrum beta-lactamases (ESBLs) in gram-negative bacterial isolates.
3.8 Statistical analysis
The data were analyzed using statistical analysis – Epi Info 7. The data collected was entered into a spreadsheet. The data were checked for missing values and completed. Analysis in terms of demographic variables, positivity in processed samples, and type of species prevalent was done using Epi Info version 7 (7.1.1.0) statistical software.
4. Results
A total of 1533 samples in the age group of fewer than five years suspected of BSIs were received in the Department of Microbiology, IGMC, Shimla. Among them, 963 (62.8%) were male, while 570 (37.2%) were female (Figure 1).
Among the total of 1533 samples, 604 (39.40%) were found positive in culture, 898 (58.58%) were negative, and 31 (2.02%) were having contaminants (Figure 2).
Among the 604 positive cases, 390 (64.6%) were males, while 214 (35.4%) were females (Table 1).
Table 2 shows gram-positive isolates.
Figure 3 shows the incidence of gram-positive isolates.
Table 3 and Figure 4 show gram-negative isolates.
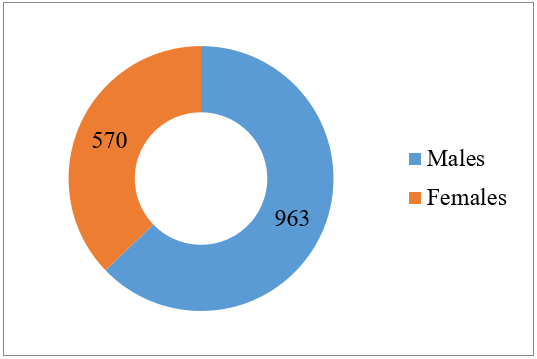 Figure 1: Gender distribution of < 5 years.
Figure 1: Gender distribution of < 5 years.
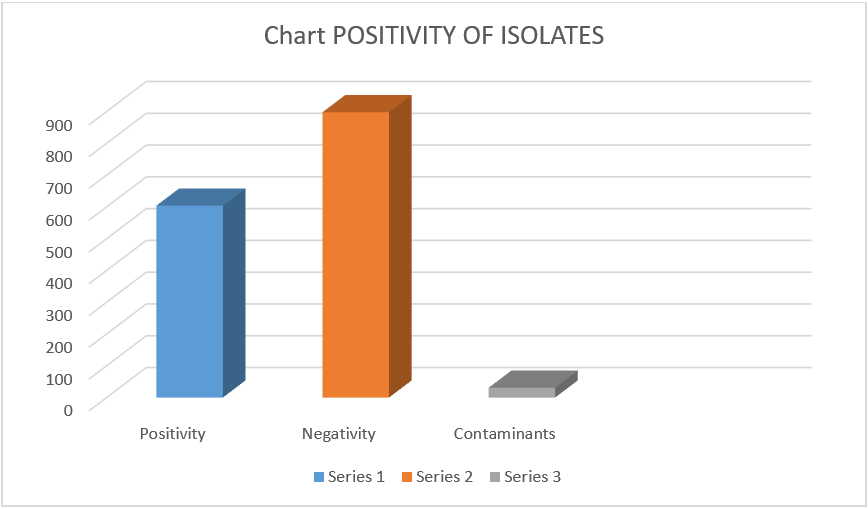
Figure 2: Distribution of samples based on the positivity of isolates.
| Culture results | Total |
| Positive | Negative | Contaminants | |
| Gender | Male | Count | 390 | 558 | 15 | 963 |
| % | 64.6% | 62.1% | 48.4% | 62.8% |
| Female | Count | 214 | 340 | 16 | 570 |
| % | 35.4% | 37.9% | 51.6% | 37.2% |
Total | Count | 604 | 898 | 31 | 1533 |
| % | 100.0% | 100.0% | 100.0% | 100.0% |
Table 1: Gender distribution of culture-positive and negative cases. Prolonged bacteremia was more common in methicillin-resistant S. aureus than in methicillin-susceptible S. aureus. Among the 604 positive cases, 390 (64.6%) were males, while 214 (35.4%) were females.
| S. aureus | 98 (16.22%) |
| Coagulase-negative Staphylococcus species | 65 (10.76%) |
| Group B Streptococcus | 30 (4.96%) |
| S. pneumoniae | 27 (4.47%) |
Table 2: Gram-positive isolates.
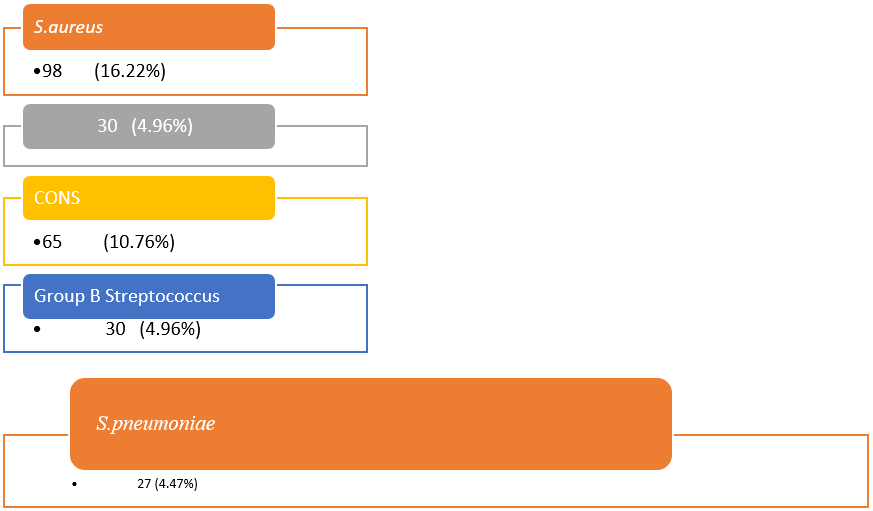 Figure 3: Distribution of culture-positive gram-positive cocci. Prolonged bacteremia was more common in methicillin-resistant S. aureus than in methicillin-susceptible S. aureus.
Figure 3: Distribution of culture-positive gram-positive cocci. Prolonged bacteremia was more common in methicillin-resistant S. aureus than in methicillin-susceptible S. aureus.
| E. coli | 80 (13.24%) |
| K. pneumoniae | 70 (11.58%) |
| P. aeruginosa | 68 (11.25%) |
| Salmonella typhi | 50 (8.27%) |
| Citrobacter koseri | 18 (2.98%) |
| Acinetobacter baumannii | 12 (1.98%) |
| Non-fermenter group of organisms | 86 (14.23%) |
Table 3: Gram-negative isolates.
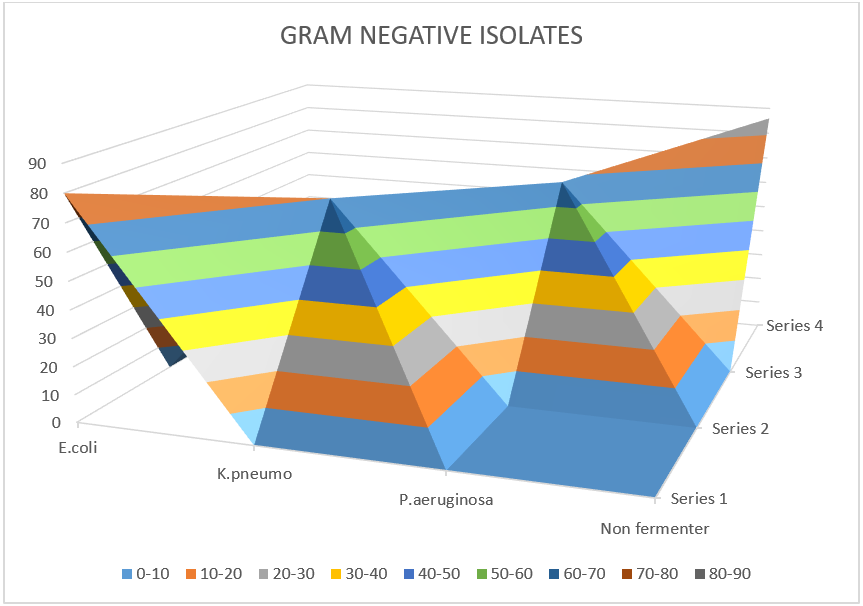 Figure 4: Gram-negative isolates.
Figure 4: Gram-negative isolates.
S. aureus 98 (16.22%) was the highest among positive isolates. Coagulase-negative Staphylococcus species 65 (10.76%), group B Streptococcus 30 (4.96%), S. pneumoniae 27 (4.47%).
E. coli 80 (13.24%) was the highest among gram-negative isolates. K. pneumoniae 70 (11.58%), P. aeruginosa 68 (11.25%), Salmonella typhi 50 (8.27%), Citrobacter koseri 18 (2.98%), Acinetobacter baumannii 12 (1.98%) and a group of organisms without fermentation 86 (14.23%).
Complications were more commonly present in patients with bacteremia > or = 4 days compared to patients with bacteremia < 4 days. The complication rate was lesser in patients who had the catheter removed < 4 days when compared to patients whose catheter was removed > or = 4 days after infection or not removed or changed.
Bacteria, viruses, or fungi that spread through the bloodstream cause BSIs. Some blood infections are relatively harmless, while others are life-threatening. The pathogens that cause these infections may come from outside the body or from inside the body where they normally live, that is, they are activated [12].
In the current study, a total of 1533 samples in the age group of < 5 years and suspected of BSI were received in the Department of Microbiology, IGMC, Shimla. Among the 963 (62.8%) were male, while 570 (37.2%) were female. Staphylococcus aureus and coagulase-negative staphylococci (CoNS) are some of the common gram-positive isolates. The virulence of CoNS organisms is relatively lower, but they can cause significant infections of the bloodstream and other sites. Risk factors for these infections include the presence of invasive devices (such as catheters, central venous lines, and intravascular catheters) and immune-depressed states. It is very difficult to distinguish true infection from contamination. True bacteremia of Staphylococcus aureus is relatively severe and has significant complications even with appropriate therapy. Streptococcus pyogenes is an aerobic gram-positive coccus and a common cause of pharyngitis and other associated invasive complications. When we consider gram-negative bacteria, they are generally associated with hospital-acquired infections and catheter-related invasive devices. Pseudomonas aeruginosa is one of the most commonly implicated gram-negative aerobic bacilli in the differential diagnosis of hospital-acquired infections. Pseudomonas aeruginosa is one of the most commonly considered gram-negative aerobic bacilli in the differential diagnosis of several probable gram-negative infections, especially in the immunosuppressed hosts, antibiotic-resistant, complicating the selection of appropriate therapy, and a significant mortality rate. E. coli meningitis, early in the course, can present without signs of central nervous system involvement. Some other gram-negative bacilli, such as Citrobacter koseri and Salmonella species, may cause brain abscesses in infants with meningitis. Salmonella are motile gram-negative bacilli that infect or colonize a wide range of mammalian hosts. They cause several infections in humans, including gastroenteritis, enteric fever, endovascular infection, and focal or localized infections like osteomyelitis or abscess.
Among the total of 1533 samples, 604 (39.40%) were positive in culture, 898 (58.58%) were negative, and 31 (2.02%) were contaminants. Among the 604 positive cases, 390 (64.6%) were male, while 214 (35.4%) were female. The rate of bacteria isolated from the culture of patients in the current study was relatively high (39.40%) compared with studies conducted in other parts of the world. The culture positivity rates documented in other studies similar to our settings conducted were 48.9% [13], 20.2% [14], 18.7% [15], and 16.6% [16].
5. Discussion
BSIs can be serious for any child, but the risk of worse outcomes is greater in children with premature births, underdeveloped immune systems, urinary tract infections, and illnesses like human immunodeficiency virus (HIV). There are many types of BSIs, including catheter-related infections (from the use of tubes placed in veins), HIV, Salmonella typhi, sepsis, Staphylococcus aureus, infective endocarditis, and those with secondary bacteremia due to focal infections, including abscesses, osteomyelitis, urinary tract infections, or pneumonia. BSI is a major cause of morbidity and mortality despite the availability of broad-spectrum and effective antimicrobials and major advances in supportive care [1, 7]. Standard empirical therapy protocols are not implemented, and increasing the prescription of broad-spectrum antibiotics is leading to toxic side effects harmful to patients and subsequently leading to failure of treatment. The most threatening is the detection of BSIs in patients undergoing treatment in critical care units, as it is predominantly nosocomial in origin, these infections are very difficult to treat due to the presence of ESBLs in gram-negative bacteria and are associated with increased failure of therapy and subsequent mortality but can be prevented by strict aseptic precautions. The increased carbapenem resistance is beginning to leave infectious disease clinicians with fewer alternatives [17].
The prevailing pattern of causative etiological agents and their sensitivity pattern is very important as it helps in the selection of specific and effective antibiotic(s) for treating the index case. It also helps to formulate an institutional policy regarding the selection of antibiotics at the time of admission in neonates admitted with suspected sepsis. This helps in preventing the misuse of antibiotics and the emergence of antibiotic resistance [16].
If the child has a blood infection, intravenous (IV) antibiotics that fight against a wide range of germs are the first course of treatment. They should be given in a combination that has a synergistic action to overcome both innate and acquired mechanisms of resistance of bacteria. Once blood cultures identify a specific disease-causing germ, the child receives more targeted antibiotics [1, 2, 7].
6. Conclusion
Pediatric sepsis criteria are not that accurate for term newborns and are not revised for preterm newborns for whom the developmental stage influences divergences are associated with impaired host immune response. Therefore, specific unanimity is needed in definitions for both term and preterm neonates. Such agreement of definitions is very much required for the analysis of observational studies, future training of clinicians, and the implementation of clinical trials in neonates. There was quite high positivity in culture in the under-five-year age group. Positivity was significantly high in males as compared to females. The majority of these BSIs were likely to have been transmitted from the environment. Improvement of hygienic precautions and implementation of BSI surveillance is recommended to decrease septicemia morbidity and mortality among hospitalized under five-year children. It is essential to administer appropriate empirical synergistic antimicrobial agents early and appropriately to treat preschool children with BSI.
References
- Okeke IN, Klugman KP, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis. 2005;5(9):568-80.
- Reynolds R, Potz N, Colman M, et al. Antimicrobial susceptibility of the pathogens of bacteraemia in the UK and Ireland 2001-2002: the BSAC Bacteraemia Resistance Surveillance Programme. J Antimicrob Chemother. 2004;53(6):1018-32.
- Diekema DJ, Beekmann SE, Chapin KC, et al. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41(8):3655-60.
- Petti CA, Polage CR, Quinn TC, et al. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42(3):377-82.
- Penno EC, Baird SJ, Crump JA. Cost-effectiveness of surveillance for bloodstream infections for sepsis management in low-resource settings. Am J Trop Med Hyg. 2015;93(4):850-60.
- Bard JD, TeKippe, et al. Diagnosis of Bloodstream Infections in Children. J Clin Microbiol. 2016;54(6):1418-1424.
- Larru B, Gong W, Vendetti N, et al. Bloodstream Infections in Hospitalized Children: Epidemiology and Antimicrobial Susceptibilities. Pediatr Infect Dis J. 2016;35(5):507-10.
- Leekha S, Terrell CL, Edson RS. General Principles of Antimicrobial Therapy. Mayo Clin Proc. 2011;86(2):156-167.
- Seifert H and Wisplinghoff H. “Bacteriology General”. In: Borriello SP, Murray RP, Funke G editors. Topley, and Wilsons Microbiology and Microbiological infections. 10th ed 1 (2001): 509-516.
- Young LS. “Sepsis syndrome”. In: Mandell GL, Mandell JE, Benett JE, Dolin R editors. Principles and practice of infectious diseases, 5thed Churchill Living stone New York (2005): 806-819.
- Wattal C, Raveendran R, Goel N, et al. Ecology of blood stream infection and antibiotic resistance in intensive care unit at a tertiary care hospital in North India. Braz J Infect Dis. 2014;18(3):245-51.
- Bloodstream infection. Riley Children’s Health.
- Meremikwu MM, Nwachukwu CE, Asuquo AE. Bacterial isolates from blood cultures of children with suspected septicaemia in Calabar, Nigeria. BMC Infect Dis. 2005;5:110.
- Arora U, Devi P. Bacterial profile of bloodstream infections and antibiotic resistance pattern of isolates. J K Sci. 2007;9:186-190.
- Murthy DS, Gyaneshwari M. Blood cultures in pediatric patients: A study of clinical impact. Indian J Med Microbiol. 2007;25(3):220-4.
- Qureshi M, Aziz F. Prevalence of microbial isolates in blood cultures and their antimicrobial susceptibility profiles. Biomedica. 2011;27:136-9.
- Pankaj Katoch. Bacteriological Profile and Antibiotic Sensitivity Pattern In Early Onset Sepsis and Late-Onset Sepsis and Comparison of ampicillin gentamicin combination with regard to 3rd generation cephalosporins for empirical treatment initiation. Int Acad J Applied BioMed Sci. 2021;2(4):12-16.




