Leishmaniasis, a disease caused by various species of the Leishmania parasite and primarily transmitted by female sandflies, poses a significant health concern in tropical and subtropical regions. This review explores recent advancements in understanding the disease, its transmission, and available treatment options. Despite the availability of treatment options such as pentavalent antimony, including sodium stibogluconate and meglumine antimoniate, their efficacy is compromised by severe side effects and the emergence of resistant parasites. The necessity for alternative therapeutics has increased interest in plant-derived compounds with anti-parasitic properties. Notable examples include Artemisia annua, Peschiera australis, Piper aduncum, and Physalis angulata, which have demonstrated promising efficacy against Leishmania parasites. However, developing effective treatments faces challenges, including the need for more stable and potent drugs. While medicinal plants offer potential solutions, their use requires caution due to possible adverse effects and interactions, particularly in vulnerable populations like children and pregnant women. Nevertheless, medicinal plants continue to be explored as valuable resources for developing safe and cost-effective treatments for leishmaniasis. In conclusion, there is an urgent need for novel therapeutic approaches to combat leishmaniasis, highlighting the potential of medicinal plants as sources of effective anti-parasitic agents.
Leishmaniasis is prevalent in tropical and subtropical areas, and various Leishmania parasites cause the disease [1]. The Leishmania species identified are Leishmania brasiliensis (L. brasiliensis), Leishmania guyanensis (L. guyanensis), Leishmania mexicana (L. mexicana), Leishmania panamensis (L. panamensis), Leishmania amazonensis (L. amazonensis), Leishmania colombiensis (L. colombiensis), and Leishmania infantum chagasi (L. infantum chagasi) [2]. Recently, leishmaniasis was discovered to be transmitted by the bite of female sandflies [3]. There are two forms of Leishmania, promastigotes and amastigote [4]. Amastigotes are intracellular, nonmotile, rounded, and spherical forms that increase inside the phagolysosomes of phagocytic cells, such as those found in vertebrates [5].
Promastigotes are extracellular, spindle-shaped, motile, and flagellated inside sandflies. Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis that involves the skin [6]. Among developing countries, CL remains a severe health problem because of its high morbidity. Around 1.5 million cases of CL are reported annually worldwide. CL is endemic in the tropics and subtropics of the globe. A common form of leishmaniasis is CL, caused by several species of Leishmania that are rarely fatal. Anthroponotic cutaneous leishmaniasis (ACL) is caused by Leishmania tropica (L. tropica), while zoonotic cutaneous leishmaniasis (ZCL) is caused by Leishmania major (L. major) [7]. Due to the severe side effects associated with commonly used leishmanicidal drugs, low doses of leishmanicidal must be used to prevent the appearance of resistance [8].
About 90% of CL cases exist in eight countries, including Iran, Brazil, Iraq, Saudi Arabia, Afghanistan, Syria, Algeria, and Peru [9]. Pentavalent antimony has been used for leishmaniasis since 1959, but it has some disadvantages, such as side effects, toxicity, and prolonged injection [10]. The development of resistant parasites has decreased the efficacy of pentavalents. Sodium stibogluconate (Pentecostal), pentamidine (Pentacarinat), and meglumine antimoniate (Glucantime) are commonly used to treat leishmaniasis, but they require longer injections to be effective [11]. Pentamidine and amphotericin B (AmB) must be prolonged and parenterally administered and are highly toxic, which reduces therapeutic compliance and stimulates the development of new alternatives. There seems to be no progress in vaccine development, and chemotherapy is currently the only treatment option. As a result, there is an urgent need for more effective, stable drugs to be developed that are more effective and possess improved features to replace or supplement the current medicines. Plant extracts are expected to become valuable sources of new medicinal treatment [12]. Recently, plant compounds with anti-parasitic properties have been discovered. These compounds include Artemisia annua, Peschiera (Tabernaemontana) australis, Piper aduncum, Physalis angulata, Plumbago scandens, and Kalanchoe pinnata demonstrating notable anti-parasitic efficacy [13–16]. Several bioactive and natural products show antileishmanial properties. Approximately 250,000 species of pharmaceutical plants worldwide, approximately 6% of which have been evaluated for their biological effects. A clinical study of therapeutic plant bioactivity has been conducted on less than 1%. In Iran, medicinal plants are a valuable source of pharmaceutical products [17]. Based on the adverse effects of herbal medicine, clinical data has shown that it is generally more accepted than synthetic medicines. Even though serious adverse events, such as interactions with herbal extract, have been reported. Thus, herbal extract should be used cautiously, particularly in specific situations, such as in children and during pregnancy. More than 820 types of herbal extract are produced in Iran [18]. Leishmaniasis is treated with indigenous medicinal plants in different cultures and countries. Public health programs increasingly encourage medicinal plants to develop safe and inexpensive treatments for infectious and non-infectious diseases. Chemically synthesized homologs of medicinal extracts of their components have specific potentials as antileishmanial compounds. Thus, experimental studies and clinical trials have been conducted regarding medicinal plants worldwide, particularly in Asian countries [19]. The current study aimed to review some current medical plants with antileishmanial activity.
Chemotherapy is the primary method of control and treatment for leishmaniasis. Antimonials are the primary drugs used in treatment. Economic factors are essential in choosing treatment since most areas affected by leishmaniasis are poor, so cheap antimonials are used as medication without vaccines [20]. There has been increased resistance to pentavalent antimonials in recent decades. As a result, alternative treatment options, such as miltefosine, AmB, and paromomycin, have been used [21]. The highly effective liposomal form of AmB reduces the drug’s toxic effects. However, a potential risk of resistance to AmB has been identified, and miltefosine treatment failure is frequently reported among patients. Understanding drug resistance can help manage it in the future.
In many parts of the world, there is a lack of knowledge about Leishmania antimony susceptibility, which makes it difficult to determine the frequency of parasite resistance associated with treatment failure. Considering this data in places with antimony as the only therapy available to prevent the spread of drug-resistant parasites is essential [22]. It is important to note that irregular and insufficient drug intake were significant contributors to the development of drug resistance among individuals. It is also difficult for poor patients to complete a course of treatment with other drugs due to the high cost of drugs. Considering antimonials are the only affordable drugs, it is also possible that patients with visceral leishmaniasis (VL) will develop drug resistance [23]. Despite the introduction of newer drugs, their development is relatively slow. Due to a lack of available treatments, drug development efforts for leishmaniasis have mainly focused on repurposing existing chemotherapy drugs. It also includes antimalarial (sitamaquine), antibiotic drugs (paromomycin), anticancer (miltefosine), and antifungal (amphotericin B).
However, severe problems with current treatments include high costs, which limit accessibility for developing countries, safety concerns regarding adverse effects and toxic effects, poor compliance with treatment regimens due to discomfort experienced during drug administration, treatment failures, and drug resistance [24]. Due to the widespread prevalence of antibiotic-resistant pathogens causing diseases such as leishmaniasis, the demand for novel antibiotics is rising [25]. Although bioactive compounds have been synthesized in a wide range of ways, nature remains the best source for novel bioactive compounds despite technological advances. Most antibiotics used to treat diseases are derived from the environment [26]. Most drug discovery approaches in the past decades involved isolating known natural products or analogs from habitats. The evidence strongly suggests that the marine environment contains an untapped source of naturally dynamic compounds, including antibiotics.
Adaptive metabolic enzymes have also evolved among parasites, making them ideal drug discovery and development targets. Meglumine antimoniate (glucantime) and antimonials sodium stibogluconate (pentostam) are among the chemotherapeutics available to treat leishmaniasis, but there are adverse effects associated with these bioactive. It is essential to explore natural sources of new antileishmanial chemotherapeutics with low toxicity compared to currently available drugs [27]. Several studies have shown that plant natural products have antileishmanial properties. Natural products, which contain a wide range of chemically diverse bioactive compounds, are the most essential sources of therapeutic agents for parasitic diseases. Additionally, these components are derived from microorganisms such as fungi and bacteria. Natural antileishmanial drug discovery efforts involved the identification of two new carbasugar-type metabolites from Geosmithia langdonii, a filamentous fungus isolated from cotton textiles from Assiut and Egypt [28] (Figure 1). Based on the compounds’ inhibitory concentration 50 (IC50) values, the compounds showed promising antileishmanial activity against L. donovani [29].
Historically, herbs have been used as medicines and disease treatments for centuries [30]. “Drug,” a medicinal preparation, derives from the old Dutch word droog, which means ”to dry” since plants were typically dried to be used as medicines [31]. It is documented in the first century Anno Domini (AD) by Pedanius Dioscorides in De materia medica, the first encyclopedia concerning herbs, that healing practices were divulged among the ancient civilizations of the Mediterranean and Asia [32]. The active constituents of a medicinal plant or its preparation are considered a single entity, whether or not they are known in detail. Phytomedicine is a medicinal product whose components are exclusively derived from medicinal plants [33]. More than 75 percent of vegetable material comprises inert structural constituents like cellulose and starch, which have no biological activity.
Most phytomedicines are extracted from these substances. A plant extract is a concentrated preparation in a liquid, powdered, or viscous form commonly produced by macerating or percolating dried or fresh plant parts [34]. The chemical composition of these preparations is only partially known, and these preparations contain a wide range of biological components. As a rule, therapeutic doses are usually expressed in milligrams per kilogram (mg/kg) based on the active constituents present in the extract or the amount of the extract required to produce the biological effect. Various liquid formulations of phytomedicines are available, including syrups, tinctures, medicinal essences, and essential oils. In contrast, granules, capsules, and uncoated or coated tablets are the most common solid formulations [31]. In pharmaceutical formulations, the quality of the extracts is a fundamental issue due to their chemical complexity. To ensure quality, ”marker compounds” should be defined as chemicals characteristic of the plant that may be used to identify, stabilize, standardize, and process the botanical material.
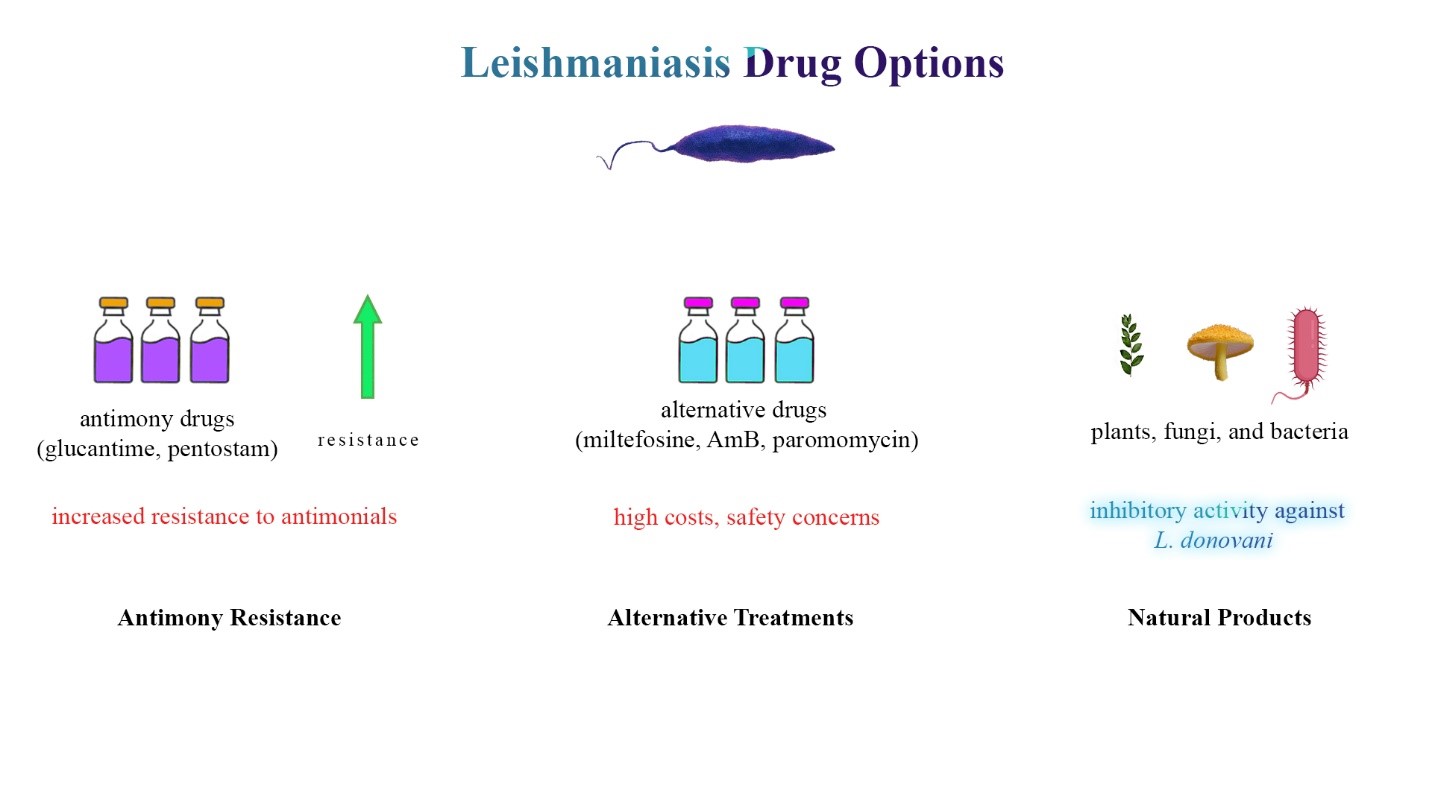
Figure 1: Leishmaniasis treatment landscape and drug discovery. This figure provides a comprehensive overview of the challenges and avenues in leishmaniasis treatment. The first panel illustrates Leishmania parasites, emphasizing the reliance on antimony drugs (glucantime, pentostam) for treatment. The second panel depicts the emergence of resistance to antimonials, influenced by economic factors leading to irregular drug intake. The third panel focuses on alternative treatments (miltefosine, AmB, paromomycin) and the slow-paced drug development pipeline, including challenges associated with current therapies. The fourth panel underscores the demand for novel antibiotics due to antibiotic-resistant pathogens and the potential of natural products, exemplified by Geosmithia langdonii, in providing new antileishmanial chemotherapeutics.
A study conducted by Maleki et al. [35] revealed that some plant extracts were effective against L. major in vivo experiments. Plant extracts increase the nitric oxide (NO) level, suppressing the parasite. According to Ezatpour et al. [36], Pistacia Shinjuku (Anacardiaceae) alcoholic extract had antileishmanial effects in vivo against L. major and L. tropica. The in vivo potential effectiveness of Artemisia annua against Leishmania was also reported in another study [37].
Using methanolic extracts from Maesa balansae leaves, a 90 percent reduction of liver amastigote burden was observed in vivo against L. infantum [38]. It has been shown by Maes et al. [39] that triterpenoid saponin extracts from the plant Maesa balansae are effective in reducing the amastigote burden in the liver by 80 % and have promising antileishmanial effect in vivo against VL. Other research revealed that ointments containing plant extracts significantly decreased ulcer size in mice infected with Leishmania and increased NO production, an essential factor in stopping parasite growth. A study conducted by Nilforoushzadeh et al. [40] evaluated the effect of extracts of Thymus vulgaris, Achillea millefolium, and propolis in mice infected with CL for decreasing the size of the ulcers. A recent study found that an alcoholic Berberis vulgaris extract improved CL symptoms in BALB/c mice and decreased lesions of CL [41]. There was also experimental evidence that Artemisia essence reduced the diameter of ulcers caused by L. major promastigote in BALB/c mice infected with promastigote infection [42]. As a result of the extract treatment, this study also found that NO levels increased in the groups treated with the extract. The Tephrosia vogelii extract significantly reduced the size and burden of parasitic lesions in BALB/c mice infected with an L. major infection [43]. A study conducted by Metwally et al. [44] showed that allicin inhibited the growth of L. major in BALB/c mice.
Kalanchoe pinnata
(Family: Crassulaceae) is a narrow, sparsely branched shrub or herb that reaches a height of one meter when in bloom. Its chemical composition includes sterols, triterpenes, and flavonoids (quercetin and kaempferol) [45]. The plant appears on the Brazilian Ministry of Public Health’s official list of medicinal plants of interest as one of the most studied species in the genus Kalanchoe [46]. Many diseases can be treated with this, including infections, rheumatism, gastric ulcers, and inflammation in general. An experimental animal model was used to evaluate the leishmanicidal effects of an aqueous extract extracted from the leaves of Kalanchoe pinnata (KP), which was infected with L. amazonensis and administered by various routes [47]. Like meglumine antimoniate, oral administration reduced lesions after 30 days of treatment. Moreover, parasite growth was suppressed by KP extract. During treatment, no toxic effects or mortality were observed in the animals. It is rich in flavonoids associated with antiallergic, antitumor, and anti-inflammatory effects [48].
KP extract also has an antileishmanial effect due to its ability to increase macrophage NO levels, which can synergistically enhance interferon-C (IFN-c) NO-inducing activity [46]. Quercitrin (quercetin-3-O-α-L-rhamnopyranoside) was isolated and, with a half-maximal inhibitory concentration (IC50) of 1.0 gram per milliliter (g/ml), was more active and less cytotoxic than the positive control pentostam. Moreover, crude extracts were more active than isolated compounds. A Brazilian patient infected with CL was clinically tested with KP aqueous extract (36 years of age, 70 kg). 215 mg/kg KP aqueous extract was administered twice daily for 14 days (equivalent to 21 mg lyophilized aqueous leaf extract) [49]. In the short course of treatment, the lesion size was significantly reduced. However, the infection returned after the interruption, and conventional treatment was given. Urea, transaminases, and alkaline phosphatase levels were not altered, and no complaints were reported. They used the KP extract as an experimental model for visceral infections caused by L. infantum syn. L. chagasi, BALB/c mice were administered 400 mg/kg KP extract daily for 30 days, and significant reductions in splenic and hepatic parasite burden were observed, similar to those administered pentecostal. The parasite-specific immunoglobulin G (IgG) serum levels were also reduced, and spleen cells could not produce interleukin 4 (IL-4), but levels of IFN-c and NO were elevated [47].
Plumbago scandens
In the Caatinga biome of Northeast Brazil, this sub-evergreen shrub measures 2–3 m long, is much branched and is along the border of secondary forests [45]. Plumbago scandens (P. scandens) roots are traditionally used as infusions for their local anesthetic properties and purgative.
In addition, plumbagin, a naphthoquinone found in most species of this genus, is effective against many types of fungi, including L. donovani, which is inhibited by its growth [50]. According to a recent study, the effects of plumbagin and its derivative 2-methoxy-1,4-naphthoquinone result from inhibitory effects on trypanothione reductase, which in turn disrupts its physiological function as an antioxidant, impairing redox homeostasis and killing the parasite [51].
Physalis angulata
It is an annual plant that grows in many countries, which are tropical and subtropical regions. Moreover, the plant is used in popular medicine for various pathologies, such as liver diseases and malaria. The study reported its activity against Trypanosoma cruzi (T. cruzi) and tumors [52]. Physalins, an essential group of secosteroids produced by this plant, have shown immunomodulatory effects in vivo and in vitro [53]. Phytoalexins B, F, or G, but not D, cause a significant decline in NO production [54]. In macrophages stimulated with lipopolysaccharide and interferon through a pathway that differs from corticosteroids. An in vitro test of physalin B, D, and F for antileishmanial activity was performed against amastigotes of L. major and L. amazonensis and L. amazonensis-infected BALB/c mice. As a result of the concentrations of physalins B and F that are not toxic for macrophages, the percentage of macrophages that were infected was reduced. Compared to mice treated only with the vehicle, mice infected with L. amazonensis received local physalin F daily, and their lesion sizes and parasite loads significantly decreased [55]. Considering that the host’s control of Leishmania infection is dependent on macrophage activation and NO production and that the immunomodulatory activity of physalins B and F inhibits the production of NO and pro-inflammatory cytokines, the beneficial effect of Physalis angulata (P. angulata) in the in vivo treatment of leishmaniasis seems to be due to a direct action of these secosteroids on the parasite or to its anti-inflammatory properties in the healing of lesions or to a combination of both these activities [56].
Piper aduncum
Plants of this family (Piperaceae) are upright shrubs with branched, jointed stems, ranging in height from 2–4 m, native to Brazil. Alcoholic and tea extracts of the leaves, fruits, and roots of Piper aduncum (P. aduncum) are generally used as carminatives, tonics, antispasmodics, and against disease of the gallbladder, liver, and spleen and gonorrhea [45]. As a result of phytochemical analysis, it has been determined that 2,6-dihydroxy-4-methoxychalcone (DMC) is present in the leaves inflorescences, which has been shown in vitro to inhibit the propagation of promastigotes and intracellular amastigotes. Promastigotes were inhibited by dichloromethane extract with a median effective dose (ED50) of 2.2 mg/ml, whereas purified DMC inhibited promastigote growth with an ED50 of 0.5 mg/ml [57]. DMC reduced the parasite load and the percentage of infected macrophages within the cell by half when tested against intracellular amastigotes at 40 and 24 g/ml, respectively [58].
The toxic effect of DMC was not observed on macrophages up to 100 lg/ml, which indicates that the compound is primarily toxic to parasites [59]. In 50 g/ml of DMC, promastigotes showed increased and more diffuse mitochondrial profiles, while the matrix and cristae patterns on the mitochondria were reduced. As a result of treating infected macrophages with 80 mg/ml of DMC, all parasites were killed [60]. When DMC was used at low doses, macrophage phagocytic function was expected. However, NO production was inhibited at low levels. It is still unknown how DMC destroys leishmania parasites. Probably through inhibition of trypanothione reductase, which controls the redox balance of trypanosomatids, it is thought to be similar to other chalcones that inhibit glutathione reductase. Studying DMC’s effects on L. amazonensis promastigotes’ sterol biosynthesis through the mevalonate pathway was also conducted. There were several changes in the sterol profile following DMC treatment, including decreased cholesterol uptake from the culture medium and decreased dehydroepisterol and episterol, L. amazonensis‘ most abundant sterols [60].
Observations indicate that DMC promotes sterol accumulation without inhibiting ergosterol biosynthesis through an unknown mechanism, resulting in altered membrane fluidity.
Peschiera (Tabernaemontana) australis
It is a small tree found in South America, Brazil, and other countries. It is an active agent against sporozoites and amastigotes of L. amazonensis that can be obtained in vitro [45]. A study conducted by Delorenzi et al. [61] identified pyronaridine as one of the most active indole alkaloids found in chloroform fraction (CLF). The growth of promastigote cells could be inhibited by coronaridine at 12.5 mg/ml to 97%. There was a 100% reduction in the development of parasites after 72 h of treatment with CLF at a concentration of 20 g/ml. This suggests irreversible damage to the ability of promastigote replication. It was found that CLF and coronaridine were effective against amastigotes in infected macrophage cultures when they were added to the culture on the first day of the culture or once a day for three days. 20 lg/ml of CLF caused the death of 98% of the parasites.
Using coronaridine and glucantime at a concentration of 20 mg/ml each, the cell death rate was 79 and 70%, respectively. As a result of exposure to CLF or pyronaridine, morphological changes in mitochondria have been observed in both amastigotes and promastigotes. The membrane cristae displayed significant swelling and disorganization in the mitochondria, while there was no change in the kinetoplast DNA. The macrophages exhibited no alterations, indicating that CLF has no toxic effects.
Another study used 18-methoxycoronaridine (18-MCOR) as a synthetic analog of pyronaridine to treat L. amazonensis-infected murine macrophages. The effects of pyronaridine and 18-MCOR were similar, but the synthetic analog had a marginally greater efficiency than the natural analog (both had IC90 of 16 mg/ml and 22 mg/ml) [62].
Artemisia annua
Artemisia annua (A. annua) is a plant in the Asteraceae family, an aromatic herb from Asia that grows in Brazil [45]. Annual erect, lupus erythematosus, and fever have been treated with the plant for centuries in India and China. Recently, new studies have shown its herbicide, insecticide, and antiulcerogenic properties in addition to its antimalarial properties. The use of A. annua needs to be documented ethnopharmacologically in Brazil. Phytochemical analysis found that the seeds contained a large volume of essential oils (about 4%), but their composition varied with the growing conditions and the plant’s chemotype. Among the oils in Annua, monoterpene concentrations, and artemisia ketone content are variable (about 64%) [63].
Camphor cineole, artemisia-alcohol, beta-pinene, and caryophyllene are standard components of the oils produced by foreign plants that have been acclimated, as well as triterpenoids, sterols, coumarin, and the sesquiterpene lactone, artemisinin, which was discovered in 1962 and revolutionized antimalarial therapy. A methanolic extract of the leaves of Artemisia indica, which contains high levels of artemisinin, was also tested against six species of Leishmania, with IC50 values ranging from 210–580 mg/ml [64]. Artemisinin was evaluated for its cytotoxicity in murine macrophages, and its viability remained unchanged at very high concentrations (0.25 mM). Observations have shown that artemisinin can induce apoptosis by depolarizing mitochondria in Leishmania. A test was carried out to determine the activity of n-hexane fractions derived from the leaves and seeds of an annua against L. donovani. It showed they have an IC50 of 6.6 and 5.0 mg/ml for intracellular amastigotes, respectively [65]. A practical antileishmanial effect was demonstrated by apoptosis, and active components such as α-amyrin acetate, β-amyrin, and derivatives of artemisinin were implicated as the principal active agents (Figure 2).
The lack of standardization in herb extracts is one of the significant challenges in treating CL with them. Several variables can affect the efficacy of herbal extracts, such as their composition and concentration of active ingredients. Future research must focus on a standardized protocol for preparing and testing herbal extracts. Generally, herbal extracts are considered safe, but their safety profile should be carefully examined, especially during prolonged use [66]. An essential component of future prospective studies should involve rigorous toxicity assessments to determine how herbal extracts may affect humans and animals over the long term. It is necessary to know that the ingredients of herbal treatments do not cause other side effects. Herbal extracts exert antileishmanial effects by various mechanisms, which require a more profound understanding [67]. These extracts are likely to contain multiple bioactive compounds, and studying their interactions with Leishmania parasites may be a valuable source of information [12]. As a result of this knowledge, treatment regimens can be optimized, and targeted therapies will be developed. Even though in vivo animal trials have shown promising results, transitioning to human trials is challenging. Conducting clinical trials with sufficient patient populations and addressing ethical considerations are vital steps for validating the efficacy of herbal extracts in treating CL [68].
 Figure 2: Medicinal plants with antileishmanial properties.
Figure 2: Medicinal plants with antileishmanial properties.
Combining herbal extracts with standard antileishmanial drugs or other herbal extracts can improve treatment outcomes and reduce resistance [46]. A future study should examine the potential for combining herbal compounds and studying their synergistic interactions. Enhancing the effectiveness of herbal extracts by increasing their bioavailability is possible. Developing new delivery systems or formulations that will enable bioactive to be absorbed and retained in the skin and affected tissues more effectively may be possible. Using genomics and proteomics to examine how the Leishmania parasite responds to herbal extracts can provide insight into potential therapeutic targets [69]. Treatments can be developed more precisely and effectively based on this knowledge. The development of robust, multifaceted therapies can be improved by examining the synergistic effects of different herbal extracts and their combinations. The effectiveness of treatment might be enhanced by different herbal extracts targeting parasites through various mechanisms. Identifying other antileishmanial plant species in endemic regions is possible by exploring Indigenous communities’ traditional knowledge and practices [70]. Researchers and local communities can help find new herbal remedies. Integrating herbal treatments into public health programs and policies can improve access to herbal treatments in endemic regions. Practitioners of healthcare professionals, traditional medicine practitioners, and policymakers must work closely together to achieve this integration. The findings of the present study suggest that herbal extracts have the potential to help in the treatment of CL [46]. However, we must address the challenges and explore prospects to maximize their clinical efficacy. The advancement of herbal extracts used in treating this debilitating disease will need to be supported by continued research, collaboration between traditional and modern medicine, and standardization. Nanotechnology and phytosomes are also perfect techniques for enhancing the power of herbal medicines (Figure 3).
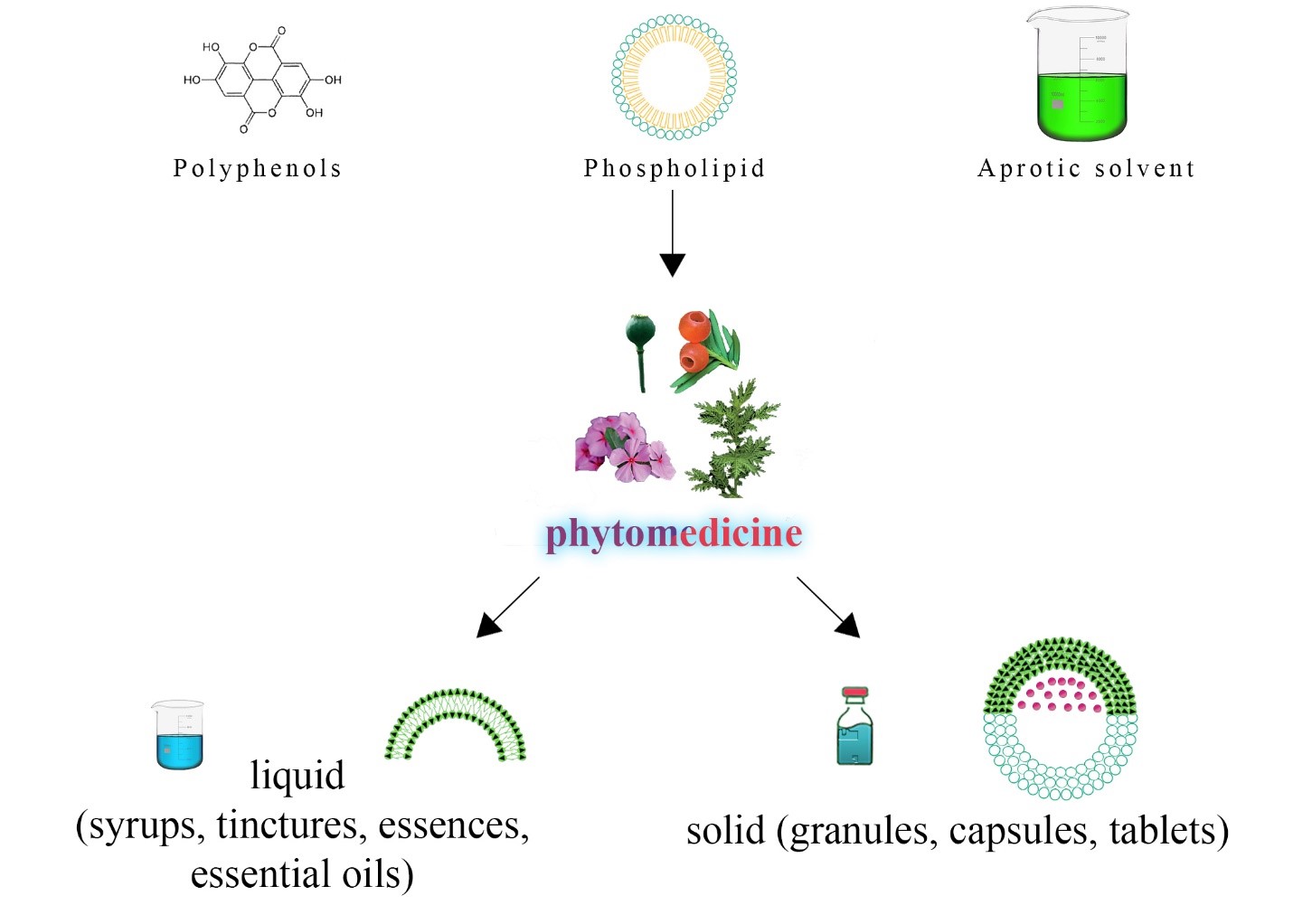 Figure 3: Components of phytomedicine. This figure illustrates the diverse forms of phytomedicine, showcasing the integration of aprotic solvent, phospholipid, and polyphenols to create phytosomes—a specialized delivery system enhancing bioavailability. The schematic includes representations of phytomedicine formulations, capturing liquid forms, such as syrups, tinctures, medicinal essences, and essential oils, and solid forms, including granules, capsules, and tablets. The figure emphasizes the versatility of pharmaceutical formulations, reflecting the complexity of phytomedicine compositions. It underscores the importance of defining “marker compounds” for quality assurance, aiding in identifying, standardizing, and processing botanical materials in pharmaceutical contexts.
Figure 3: Components of phytomedicine. This figure illustrates the diverse forms of phytomedicine, showcasing the integration of aprotic solvent, phospholipid, and polyphenols to create phytosomes—a specialized delivery system enhancing bioavailability. The schematic includes representations of phytomedicine formulations, capturing liquid forms, such as syrups, tinctures, medicinal essences, and essential oils, and solid forms, including granules, capsules, and tablets. The figure emphasizes the versatility of pharmaceutical formulations, reflecting the complexity of phytomedicine compositions. It underscores the importance of defining “marker compounds” for quality assurance, aiding in identifying, standardizing, and processing botanical materials in pharmaceutical contexts.



